Abstract
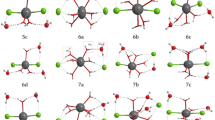
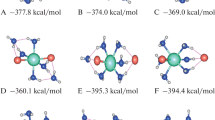
In this work, the structural details and Raman spectra of the Ca(NO3)2(H2O) n=0–10 clusters were studied by using ab initio method. The results show that the main species in the cluster is the contact ion pair (CIP) when n = 1–7. When n = 8–10, the main species changes into solvent shared ion pair (SIP) CaNO3(H2O) n …NO3 − in the bidentate form. One of the r Ca–N distances remains unchanged at ~2.95 Å, while the other one increases to more than 4.8 Å. The hydration distance r Ca–O remains at 2.42 Å. The contact between Ca2+ and NO3 leads to a red shift of the v 1–NO3 − band while the polarization of water by Ca2+ leads to a blue shift. The vibrational frequency of water molecules remains unchanged for the same types of water molecules. Hydrogen bonds are the main reason for the red shift of vibrational frequency of water molecules.




Similar content being viewed by others
References
A. T. Blades, M. Peschke, U. H. Verkerk, and P. Kebarle (2004). J. Am. Chem. Soc. 126, 11995.
Y. Inada, H. Hayashi, K. Sugimoto, and S. Funahashi (1999). J. Phys. Chem. A 103, 1401.
H. Ke, C. Linde, and J. M. Lisy (2015). J. Phys. Chem. A 119, 2037.
N. Hewish, G. Neilson, and J. Enderby (2001). J. Am. Chem. Soc. 123, 431.
F. Jalilehvand, D. Spangberg, P. Lindqvist-Reis, K. Hermansson, I. Persson, and M. Sandstro (2001). J. Am. Chem. Soc. 123, 431.
M. F. Bush, R. J. Saykally, and E. R. Williams (2005). J. Am. Chem. Soc. 127, 16599.
F. Perakis, L. D. Marco, A. Shalit, F. Tang, Z. R. Kann, T. D. Kuhne, R. Torre, M. Bonn, and Y. Nagata (2016). Chem. Rev. 116, 7590.
D. J. Miller and J. M. Lisy (2006). J. Chem. Phys. 124, 024319-1.
T. Megyes, S. Balint, E. Peter, T. Grosz, I. Bako, H. Krienke, and M. Bellissent-Funel (2009). J. Phys. Chem. B 113, 4054.
Z. Zeng, C. W. Liu, G. L. Hou, G. Feng, H. G. Xu, Y. Q. Gao, and W. J. Zheng (2015). J. Phys. Chem. A 119, 2845.
V. T. Pham and J. L. Fulton (2013). J. Chem. Phys. 138, 044201-1.
J. Fulton, S. Heald, Y. Badyal, and J. Simonson (2003). J. Phys. Chem. A 107, 4688.
T. Todorova, P. H. Hunenberger, and J. Hutter (2008). J. Chem. Theory Comput. 4, 779.
Q. Dai, J. J. Xu, H. J. Li, and H. B. Yi (2015). Mol. Phys. 133, 1.
T. G. Chang and D. E. Irish (1973). J. Phys. Chem. 77, 52.
D. W. James and M. T. Carrick (1982). J. Raman Spectrosc. 13, 115.
M. Peleg (1972). J. Phys. Chem. 76, 1019.
X. H. Li, L. J. Zhao, J. L. Dong, H. S. Xiao, and Y. H. Zhang (2008). J. Phys. Chem. B 112, 5032.
M. Eigen and K. Z. Tamm (1962). Elektrochem 66, 93.
M. Eigen and K. Z. Tamm (1962). Elektrochem 66, 107.
H. Zhang and Y. H. Zhang (2009). J. Comput. Chem. 31, 2772.
L. Jiang, T. Wende, R. Bergmann, G. Meijer, and K. R. Asmis (2010). J. Am. Chem. Soc. 132, 7398.
W. W. Rudolph and G. Irmer (2013). Dalton Trans. 42, 3919.
W. W. Rudolph, D. Fischer, G. Irmerc, and C. C. Pye (2009). Dalton Trans. 33, 6513.
W. W. Rudolph, R. Masonb, and C. C. Pye (2000). Phys. Chem. Chem. Phys. 2, 5030.
W. W. Rudolph and C. C. Pye (2002). Phys. Chem. Chem. Phys. 4, 4319.
W. W. Rudolph and G. Irmer (2013). Dalton Trans. 42, 14460.
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
C. Lee, W. Yang, and R. G. Parr (1988). Phys. Rev. B 37, 785.
R. Ditchfield, W. J. Hehre, and J. A. Pople (1971). J. Chem. Phys. 54, 724.
V. S. Bryantsev, M. S. Diallo, and W. A. Goddard (2009). J. Phys. Chem. B 112, 9709.
B. Mennucci, E. Cances, and J. Tomasi (1997). J. Phys. Chem. B 101, 10506.
J. D. Chai and M. Head-Gordon (2008). Phys. Chem. Chem. Phys. 10, 6615.
Z. Zeng, G. L. Hou, J. Song, G. Feng, H. G. Xu, and W. J. Zheng (2015). Phys. Chem. Chem. Phys. 17, 9135.
D. Rappoport and F. Furche (2010). J. Chem. Phys. 133, 134105-1.
C. N. Rowley and B. Roux (2012). J. Chem. Theory Comput. 8, 3526.
A. P. Scott and L. Radom (1996). J. Phys. Chem. 100, 16502.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox. (Gaussian, Inc., Wallingford CT, 2013). Gaussian 09, Revision C.01.
P. M. Vollmar (1963). J. Chem. Phys. 39, 2236.
H. Brintzinger and R. E. Hester (1966). Inorg. Chem. 5, 980.
R. E. Hester and W. E. L. Grossman (1966). Inorg. Chem. 5, 1308.
Acknowledgements
We thank the National Natural Science Foundation of China (Nos. 21373251, 21573268), the Natural Science Foundation of Qinghai (No. 2015-ZJ-938Q), and the Young People Fund of Qinghai University (No. 2015-QGY-7) for financial support. We also acknowledge computing resources and time in the supercomputing center of National Super Computing Center in Shenzhen.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, F., Zhou, H., Zhou, Y. et al. Ab Initio Investigation of the Micro-species and Raman Spectra in Ca(NO3)2 Solution. J Clust Sci 28, 2293–2307 (2017). https://doi.org/10.1007/s10876-017-1210-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1210-4