Abstract
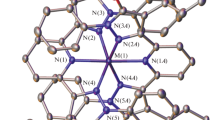
Treatment of FeCl3 with 1 equiv of 3-(pyrid-2-yl)-5-(tertbutyl)-1H-pyrazole (L) in basic methanol affords crystalline [Fe3Cl5(μ 3-O)(μ-OMe)2L2(MeOH)] in moderate yield. The compound has an unusual isosceles triangular [Fe3(μ 3-O)(μ-OMe)2]5+ core with one unbridged edge. Two of the iron(III) centres in the compound are approximately octahedral, while the third has a five-coordinate geometry. Magnetic susceptibility measurements show antiferromagnetic coupling between the iron centres, leading to a S = 3/2 magnetic ground state and S = 5/2 excited state that are almost accidentally degenerate according to simulation. An analogous reaction using Fe[ClO4]3 as starting material instead affords the low-spin iron(II) complex [FeL3][ClO4]2.




Similar content being viewed by others
References
L. Que Jr. and W. B. Tolman (2002). Angew. Chem. Int. Ed. 41, 1114.
M. H. Sazinsky and S. J. Lippard (2006). Acc. Chem. Res. 39, 558.
L.-O. Essen, S. Offermann, D. Oesterhelt, and K. Zeth in E. Bäuerlein (ed.), Biomineralization, 2nd ed (Wiley-VCH, Weinheim, Germany, 2004), pp. 119–133.
E. C. Theil, X. S. Liu, and M. Matzapetakis (2008). Met. Ions Life Sci. 4, 327.
D. Gatteschi, R. Sessoli, and A. Cornia (2000). Chem. Commun., 725.
G. Aromi and E. K. Brechin (2006). Struct. Bonding (Berlin) 122, 1.
R. Lawaczeck, M. Menzel, and H. Pietsch (2004). Appl. Organomet. Chem. 18, 506.
R. D. Cannon and R. P. White (1988). Prog. Inorg. Chem. 36, 195.
R. Celenligil-Cetin, R. J. Staples, and P. Stavropoulos (2000). Inorg. Chem. 39, 5838.
P.-G. Lassahn, V. Lozan, G. A. Timco, P. Christian, C. Janiak, and R. E. P. Winpenny (2004). J. Catal. 222, 260.
D. Ogrin, R. Colorado Jr., B. Maruyama, M. J. Pender, R. E. Smalley, and A. R. Barron (2006). Dalton Trans., 229.
G. Trettenhahn, M. Nagl, N. Neuwirth, V. B. Arion, W. Jary, P. Pöchlauer, and W. Schmid (2006). Angew. Chem. Int. Ed. 45, 2794.
S. M. Gorun, G. C. Papaefthymiou, R. B. Frankel, and S. J. Lippard (1987). J. Am. Chem. Soc. 109, 4244.
M. Hirotsu, M. Kojima, W. Mori, and Y. Yoshikawa (1999). Chem. Lett. 28, 229.
R. Bagai, S. Datta, A. Betancur-Rodriguez, K. A. Abboud, S. Hill, and G. Christou (2007). Inorg. Chem. 46, 4535.
P. Alborés and E. Rentschler (2008). Eur. J. Inorg. Chem., 4004.
R. W. Saalfrank, A. Scheurer, K. Pokorny, H. Maid, U. Reimann, F. Hampel, F. W. Heinemann, V. Schünemann, and A. X. Trautwein (2005). Eur. J. Inorg. Chem., 1383.
R. W. Saalfrank, A. Scheurer, U. Reimann, F. Hampel, C. Trieflinger, M. Büschel, J. Daub, A. X. Trautwein, V. Schünemann, and V. Coropceanu (2005). Chem. Eur. J. 11, 5843.
A. Caneschi, A. Cornia, A. C. Fabretti, D. Gatteschi, and W. Malavasi (1995). Inorg. Chem. 34, 4660.
R. W. Saalfrank, S. Trummer, H. Krautscheid, V. Schünemann, A. X. Trautwein, S. Hien, C. Stadler, and J. Daub (1996). Angew. Chem. Int. Ed. 35, 2206.
E. Bill, C. Krebs, M. Winter, M. Gerdan, A. X. Trautwein, U. Florke, H.-J. Haupt, and P. Chaudhuri (1997). Chem. Eur. J. 3, 193.
S. G. Sreerama and S. Pal (2004). Eur. J. Inorg. Chem., 4718.
H. A. Burkill, N. Robertson, R. Vilar, A. J. P. White, and D. J. Williams (2005). Inorg. Chem. 44, 3337.
X. Liu, J. A. McAllister, M. P. de Miranda, E. J. L. McInnes, C. A. Kilner, and M. A. Halcrow (2004). Chem. Eur. J. 10, 1827. and refs. therein.
L. F. Jones, K. D. Camm, C. A. Kilner, and M. A. Halcrow (2006). CrystEngComm 8, 719.
L. F. Jones, C. A. Kilner, M. P. de Miranda, J. Wolowska, and M. A. Halcrow (2007). Angew. Chem. Int. Ed. 46, 4073.
L. F. Jones, S. A. Barrett, C. A. Kilner, and M. A. Halcrow (2008). Chem. Eur. J. 14, 223.
L. F. Jones, C. A. Kilner, and M. A. Halcrow (2009). Chem. Eur. J. 15, 4667.
C. J. O’Connor (1982). Prog. Inorg. Chem. 29, 203.
W.-S. Yu, C.-C. Cheng, Y.-M. Cheng, P.-C. Wu, Y.-H. Song, Y. Chi, and P.-T. Chou (2003). J. Am. Chem. Soc. 125, 10800.
Z. Otwinowski and W. Minor (1997). Meth. Enzymol. 276, 307.
R. H. Blessing (1995). Acta Crystallogr. Sect. A 51, 33.
G. M. Sheldrick (2008). Acta Crystallogr. Sect. A 64, 112.
L. J. Barbour (2001). J. Supramol. Chem. 1, 189.
POVRAY v. 3.5, Persistence of Vision Raytracer Pty. Ltd., Williamstown, Victoria, Australia (2002). http://www.povray.org.
K. Nakamoto Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, 5th ed (Wiley Interscience, New York, 1997), p. 54.
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, and G. C. Verschoor (1984). J. Chem. Soc., Dalton Trans., 1349.
S. M. Gorun and S. J. Lippard (1991). Inorg. Chem. 30, 1625.
E. König (1987). Prog. Inorg. Chem. 35, 527.
K. H. Sugiyarto and H. A. Goodwin (1988). Aust. J. Chem. 41, 1645.
L. S. Harimanow, K. H. Sugiyarto, D. C. Craig, M. L. Scudder, and H. A. Goodwin (1999). Aust. J. Chem. 52, 109.
T. Kajiwara and T. Ito (2000). Angew. Chem. Int. Ed. 39, 230.
M. D. Fryzuk, D. B. Leznoff, E. S. F. Ma, S. J. Rettig, and V. G. Young Jr. (1998). Organometallics 17, 2313.
A. Shrivastav, N. K. Singh, and S. M. Singh (2002). Bioorg. Med. Chem. 10, 887.
G. Mund, R. J. Batchelor, R. D. Sharma, C. H. W. Jones, and D. B. Leznoff (2002). J. Chem. Soc., Dalton Trans., 136.
V. C. Gibson, S. K. Spitzmesser, A. J. P. White, and D. J. Williams (2003). Dalton Trans., 2718.
R. Weiss, A. Gold, and J. Terner (2006). Chem. Rev. 106, 2550.
Acknowledgements
The authors thank Dr. H. J. Blythe (University of Sheffield) for the magnetic susceptibility data, and Professor Eric McInnes and Drs. Ruth Edge and Joanna Wolowska (University of Manchester) for preliminary EPR measurements on 1. This work was supported by the Leverhulme Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Malcolm Chisholm.
Rights and permissions
About this article
Cite this article
Jones, L.F., Kilner, C.A. & Halcrow, M.A. A Trinuclear Iron(III) Compound with an Unusual T-Shaped [Fe3(μ 3-O)]7+ Core. J Clust Sci 21, 279–290 (2010). https://doi.org/10.1007/s10876-010-0283-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-010-0283-0