Abstract
Purpose
Female carriers with X-linked chronic granulomatous disease (XL-CGD) who have < 10% reactive oxygen species (ROS) production due to profound X-chromosome inactivation (XCI or lyonization) are more susceptible to infections. We assessed ROS production in Taiwanese female carriers with XL-CGD to investigate whether the level of ROS correlated to their clinical features of infection, autoimmunity, and autoinflammation.
Methods
Clinical course, ROS production, flavocytochrome b558 (Cyto b558) expression, and genetic analysis in carriers were investigated after identifying their index cases between 2004 and 2019.
Results
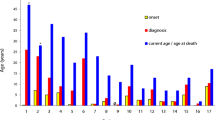
A total of 19 mothers (median 27 years; range 25–60 years) and three of four girls (range 4–6 years) relative to 22 male index XL-CGD cases from 19 unrelated families were enrolled. Approximately half (8/19, 42%) of the mothers had novel one-allele mutations. Twenty-two of the 23 females were carriers. One carrier with de novo [Arg290X]CYBB who suffered from refractory salmonella sepsis and chorioretinitis as an XL-CGD phenotype had extreme XCI, absent Cyto b558 expression, and only 8% ROS production. The remaining carriers had bimodal patterns of Cyto b558 expressions (median 40.2%, 26.8–52.4%) and ROS production (38.3%, range 28.2–54.2%) sufficient to prevent significant infections, although neck lymphadenitis recurred in one mother and sister who had ROS expressions of 28.2% and 38.0%, respectively. However, none of the carriers had manifestations of autoimmunity or autoinflammation (e.g., photosensitivity, aphthous stomatitis, or joint disorders), of which each was seen in approximately one-third of XL-CGD carriers from the Western world.
Conclusion
One carrier had undetectable Cyto b558 expression and an extremely low ROS production, and consequently presented with an XL-CGD phenotype. One mother and her daughter experienced recurrent neck lymphadenitis despite having sufficient ROS production. Significant autoimmunity/autoinflammation did not develop in any of the carriers. Studies with a longer follow-up period are needed to validate our findings.





Similar content being viewed by others

References
Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore). 2000;79(3):170–200. https://doi.org/10.1097/00005792-200005000-00004.
Thomas DC, Charbonnier LM, Schejtman A, et al. EROS/CYBC1 mutations: decreased NADPH oxidase function and chronic granulomatous disease. J Allergy Clin Immunol. 2019;143(2):782-785.e1. https://doi.org/10.1016/j.jaci.2018.09.019.
Lacerda-Pontes R, Gomes LN, Albuquerque RS, Soeiro-Pereira PV, Condino-Neto A. The extended understanding of chronic granulomatous disease. Curr Opin Pediatr. 2019;31(6):869–73. https://doi.org/10.1097/MOP.0000000000000830.
Cazzola M, Sacchi F, Pagani A, et al. X-linked chronic granulomatous disease in an adult woman. Evidence for a cell selection favoring neutrophils expressing the mutant allele. Haematologica. 1985;70(4):291–5.
Jirapongsananuruk O, Malech HL, Kuhns DB, et al. Diagnostic paradigm for evaluation of male patients with chronic granulomatous disease, based on the dihydrorhodamine 123 assay. J Allergy Clin Immunol. 2003;111(2):374–9. https://doi.org/10.1067/mai.2003.58.
Pulvirenti F, Sangerardi M, Plebani A, et al. Health-related quality of life and emotional difficulties in chronic granulomatous disease: data on adult and pediatric patients from Italian Network for Primary Immunodeficiency (IPINet). J Clin Immunol. 2020;40(2):289–98. https://doi.org/10.1007/s10875-019-00725-1.
Kohn DB, Booth C, Kang EM, et al. Lentiviral gene therapy for X-linked chronic granulomatous disease. Nat Med. 2020;26(2):200–6. https://doi.org/10.1038/s41591-019-0735-5.
El Kassar N, Hetet G, Brière J, Grandchamp B. X-chromosome inactivation in healthy females: incidence of excessive lyonization with age and comparison of assays involving DNA methylation and transcript polymorphisms. Clin Chem. 1998;44(1):61–7.
Buescher ES, Alling DW, Gallin JI. Use of an X-linked human neutrophil marker to estimate timing of lyonization and size of the dividing stem cell pool. J Clin Invest. 1985;76(4):1581–4. https://doi.org/10.1172/JCI112140.
Curnutte JT, Hopkins PJ, Kuhl W, Beutler E. Studying X inactivation. Lancet. 1992;339(8795):749. https://doi.org/10.1016/0140-6736(92)90653-k.
Hauck F, Koletzko S, Walz C, et al. Diagnostic and treatment options for severe IBD in female X-CGD carriers with non-random X-inactivation. J Crohns Colitis. 2016;10(1):112–5. https://doi.org/10.1093/ecco-jcc/jjv186.
Curnutte JT. Molecular basis of the autosomal recessive forms of chronic granulomatous disease. Immunodefic Rev. 1992;3(2):149–72.
Smith RM, Curnutte JT. Molecular basis of chronic granulomatous disease. Blood. 1991;77(4):673–86.
Roos D, de Boer M, Kuribayashi F, et al. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87(5):1663–81.
Winkelstein JA, Marino MC, Johnston RB Jr, et al. Chronic granulomatous disease Report on a national registry of 368 patients. Medicine (Baltimore). 2000;79(3):155–69. https://doi.org/10.1097/00005792-200005000-00003.
Disteche CM, Berletch JB. X-chromosome inactivation and escape. J Genet. 2015;94(4):591–9. https://doi.org/10.1007/s12041-015-0574-1.
Lee WI, Torgerson TR, Schumacher MJ, Yel L, Zhu Q, Ochs HD. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood. 2005;105(5):1881–90. https://doi.org/10.1182/blood-2003-12-4420.
van Pelt LJ, van Zwieten R, Weening RS, Roos D, Verhoeven AJ, Bolscher BG. Limitations on the use of dihydrorhodamine 123 for flow cytometric analysis of the neutrophil respiratory burst. J Immunol Methods. 1996;191(2):187–96. https://doi.org/10.1016/0022-1759(96)00024-5.
Burritt JB, Foubert TR, Baniulis D, et al. Functional epitope on human neutrophil flavocytochrome b558. J Immunol. 2003;170(12):6082–9. https://doi.org/10.4049/jimmunol.170.12.6082.
Lee WI, Jaing TH, Hsieh MY, Kuo ML, Lin SJ, Huang JL. Distribution, infections, treatments and molecular analysis in a large cohort of patients with primary immunodeficiency diseases (PIDs) in Taiwan. J Clin Immunol. 2006;26(3):274–83. https://doi.org/10.1007/s10875-006-9013-7.
Uehara S, Hashiyada M, Sato K, Sato Y, Fujimori K, Okamura K. Preferential X-chromosome inactivation in women with idiopathic recurrent pregnancy loss. Fertil Steril. 2001;76(5):908–14. https://doi.org/10.1016/s0015-0282(01)02845-x.
Global variome shared LOVD CYBB (cytochrome b-245, beta polypeptide) https://databases.lovd.nl/shared/variants/CYBB.
Roos D, Kuhns DB, Maddalena A, et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol Dis. 2010;45(3):246–65. https://doi.org/10.1016/j.bcmd.2010.07.012.
Marciano BE, Zerbe CS, Falcone EL, et al. X-linked carriers of chronic granulomatous disease: illness, lyonization, and stability. J Allergy Clin Immunol. 2018;141(1):365–71. https://doi.org/10.1016/j.jaci.2017.04.035.
López-Hernández I, Guzmán-Martínez MN, Medina-Vera I, et al. Clinical manifestations in carriers of X-linked chronic granulomatous disease in Mexico. J Investig Allergol Clin Immunol. 2019;29(2):134–6. https://doi.org/10.18176/jiaci.0343.
Battersby AC, Braggins H, Pearce MS, et al. Inflammatory and autoimmune manifestations in X-linked carriers of chronic granulomatous disease in the United Kingdom. J Allergy Clin Immunol. 2017;140(2):628-630.e6. https://doi.org/10.1016/j.jaci.2017.02.029.
van den Berg JM, van Koppen E, Åhlin A, et al. Chronic granulomatous disease: the European experience. PLoS ONE. 2009;4(4).
Hasui TS. Chronic granulomatous disease in Japan: incidence and natural history. The Study Group of Phagocyte Disorders of Japan. Pediatr Int. 1999;41(5):589–93.
Goldblatt D, Butcher J, Thrasher AJ, Russell-Eggitt I. Chorioretinal lesions in patients and carriers of chronic granulomatous disease. J Pediatr. 1999;134(6):780–3. https://doi.org/10.1016/s0022-3476(99)70299-4.
Rösen-Wolff A, Soldan W, Heyne K, Bickhardt J, Gahr M, Roesler J. Increased susceptibility of a carrier of X-linked chronic granulomatous disease (CGD) to Aspergillus fumigatus infection associated with age-related skewing of lyonization. Ann Hematol. 2001;80(2):113–5. https://doi.org/10.1007/s002770000230.
Lun A, Roesler J, Renz H. Unusual late onset of X-linked chronic granulomatous disease in an adult woman after unsuspicious childhood. Clin Chem. 2002;48(5):780–1.
Anderson-Cohen M, Holland SM, Kuhns DB, et al. Severe phenotype of chronic granulomatous disease presenting in a female with a de novo mutation in gp91-phox and a non familial, extremely skewed X chromosome inactivation. Clin Immunol. 2003;109(3):308–17. https://doi.org/10.1016/j.clim.2003.08.002.
Chollet-Martin S, Lopez A, Gaud C, et al. Severe X-linked chronic granulomatous disease in two unrelated females. Eur J Pediatr. 2007;166(2):153–9. https://doi.org/10.1007/s00431-006-0211-3.
Migliavacca M, Assanelli A, Ferrua F, et al. Pioglitazone as a novel therapeutic approach in chronic granulomatous disease. J Allergy Clin Immunol. 2016;137(6):1913-1915.e2. https://doi.org/10.1016/j.jaci.2016.01.033.
Hui X, Liu D, Wang W, et al. Low-dose pioglitazone does not increase ROS production in chronic granulomatous disease patients with severe infection. J Clin Immunol. 2020;40(1):131–7. https://doi.org/10.1007/s10875-019-00719-z.
Acknowledgements
The authors wish to thank all of the patients and their families for their kind cooperation, as well as their physicians for the referrals.
Funding
This study is funded by the Chang-Gung Medical Research Progress (Grant CMRPG 3G0441 and CMRPG 3K0261), the National Science Council (Grants NSC 102–2314-B-182A-039-MY3, MOST 106–2314-B-182A-147, MOST 109–2314-B-182A-109, NMRPG 3G0381, and PMRPG 3H0051), and the Taiwan Foundation for Rare Disorders (TFRD).
Author information
Authors and Affiliations
Contributions
Wu CY, Chen YC, and Lee WI carried out the molecular genetic studies, analyzed the sequence alignment, and drafted the manuscript. Wu CY, Lee WI, and Chiu CH performed the immunoassays. Lee WI and Chiu CH designed the study and the statistical analysis. Chen LC, Yao TC, Ou LS, Jaing TH, Chen SH, and Huang JL participated in the study to care for the critical patients. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOC 83 kb)
Rights and permissions
About this article
Cite this article
Wu, CY., Chen, YC., Lee, WI. et al. Clinical Features of Female Taiwanese Carriers with X-linked Chronic Granulomatous Disease from 2004 to 2019. J Clin Immunol 41, 1303–1314 (2021). https://doi.org/10.1007/s10875-021-01055-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-021-01055-x