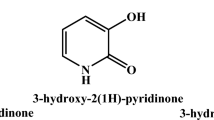
Abstract
A series of heterotrimetallic manganese-lanthanide-sodium dimer metallacrowns has been synthesized and characterized by single-crystal X-ray analysis: {LnNa[12-MCMn(III)N(shi)-4]}2(iph)4, where LnIII = La (1), Ce (2), Pr (3), Nd (4), Sm (5), Eu (6), Gd (7), Tb (8), Dy (9), Ho (10), Er (11), Tm (12), Yb (13), Lu (14), and Y (15); MC is metallacrown; shi3− is salicylhydroximate; and iph2− is isophthalate. The manganese(III) ions and shi3− ligands generate the 12-MC-4 framework with one LnIII and Na+ ion bound to each [12-MCMn(III)N(shi)-4] on opposite sides of the central MC cavity. The carboxylate groups of the isophthalate ligands bridge between the central LnIII ion and each ring MnIII ion, and the meta-arrangement of the carboxylate groups joins two LnNa[12-MCMn(III)N(shi)-4] units together to form the dimer through the LnIII ions, which reside on the interior of the molecule. The identity of the central LnIII ion slightly impacts the size the [12-MCMn(III)N(shi)-4] framework. As the crystal radius of the LnIII ion increases from LuIII (1.02 Å) to LaIII (1.19 Å), the 12-MC-4 framework expands to accommodate the larger LnIII ion as the MC cavity increases in size (0.53 Å for LuIII to 0.58 Å for LaIII) and the average cross cavity MnIII-MnIII and oxime oxygen-oxime oxygen distances also increase (MnIII-MnIII distances: 6.48 Å for LuIII to 6.52 Å for LaIII; Ooxime-Ooxime distances: 3.66 Å for LuIII to 3.75 Å for LaIII). In addition, the larger LnIII ions reside further from the MC cavity as indicated by the LnIII-oxime oxygen mean plane (OoxMP) distance. The LnIII-OoxMP distance steadily decreases from LaIII (1.7527(12) Å) to LuIII (1.5575(15) Å).
Graphic Abstract
The complex {LaNa[12-MCMn(III)N(shi)-4]}2(iph)4(DMF)6(H2O)2 is a dimer of [12-MC-4] molecules linked by four isophthalate anions





Similar content being viewed by others
References
Mezei G, Zaleski CM, Pecoraro VL (2007) Structural and Functional Evolution of Metallacrowns. Chem Rev 107:4933–5003
Lah MS, Pecoraro VL (1989) Isolation and Characterization of {MnII[MnIII(salicylhydroximate)]4(acetate)2(DMF)6}·2DMF: An Inorganic Analogue to M2+(12-crown-4). J Am Chem Soc 111:7258–7259
Lah MS, Kirk ML, Hatfield W, Pecoraro VL (1989) The Tetranuclear FeIII[FeIII(salicylhydroximate)(MeOH)(acetate)]3 is an Analogue of M3+ (9-crown-3). J Chem Soc Chem Commun 1606–1608
Lah MS, Pecoraro VL (1991) A functional analogy between crown ethers and metallacrowns. Inorg Chem 30:878–880
Azar MR, Boron TT III, Lutter JC, Daly CI, Zegalia KA, Nimthong R, Ferrence GM, Zeller M, Kampf JW, Pecoraro VL, Zaleski CM (2014) Controllable Formation of Heterotrimetallic Coordination Compounds – Systematically Incorporating Lanthanide and Alkali Metal Ions into the Manganese 12-Metallacrown-4 Framework. Inorg Chem 53:1729–1742
Travis JR, Zeller M, Zaleski CM (2015) Crystal structure of tetraaqua(dimethylformamide) tetrakis(μ-N,2-dioxidobenzene-1-carboximidato)tetrakis(μ-trimethylacetato)tetramanganese(III)sodiumyttrium–dimethylformamide–water (1/8.04/0.62). Acta Cryst E71:1300–1306
Travis JR, Zeller M, Zaleski CM (2016) Facile carboxylate ligand variation of heterotrimetallic 12-metallacrown-4 complexes. Polyhedron 114:29–36
Boron TT III, Lutter JC, Daly CI, Chow CY, Davis AH, Nimthong-Roldán A, Zeller M, Kampf JW, Zaleski CM, Pecoraro VL (2016) The nature of the bridging anion controls the single-molecule magnetic properties of DyX4M 12-metallacrown-4 complexes. Inorg Chem 55:10597–10607
Manickas EC, Zeller M, Zaleski CM (2020) Crystal structure of two heterotrimetallic dysprosium-manganese-sodium 12-metallacrown-4 complexes with the bridging ligands 3-hydroxybenzoate and 4-hydroxybenzoate. Acta Cryst E76:1213–1221
Chow CY, Eliseeva SV, Trivedi ER, Nguyen TN, Kampf JW, Petoud S, Pecoraro VL (2016) Ga3+/Ln3+ metallacrowns: a promising family of highly luminescent lanthanide complexes that covers visible and near-infrared domains. J Am Chem Soc 138:5100–5109
Travis JR, Smihosky AM, Kauffman AC, Ramstrom SE, Lewis AJ, Nagy SG, Rheam RE, Zeller M, Zaleski CM (2020) Syntheses and Crystal Structures of Two Classes of Aluminum-Lanthanide-Sodium Heterotrimetallic 12-Metallacrown-4 Compounds: Individual Molecules and Dimers of Metallacrowns. J Chem Crystallogr. https://doi.org/10.1007/s10870-020-00861-2
Nguyen TN, Chow CY, Eliseeva SV, Trivedi ER, Kampf JW, Martinić I, Petoud S, Pecoraro VL (2018) One-step assembly of visible and near-infrared emitting metallacrown dimers using a bifunctional linker. Chem Eur J 24:1031–1035
Lutter JC, Lopez Bermudez BA, Nguyen TN, Kampf JW, Pecoraro VL (2019) Functionalization of luminescent lanthanide-gallium metallacrowns using copper-catalyzed alkyne-azide cycloaddition and thiol-maleimide Michael addition. J Inorg Biochm 192:119–125
Lutter JC, Eliseeva SV, Collet G, Martinić I, Kampf JW, Schneider B, Carichner A, Sobilo J, Lerondel S, Petoud S, Pecoraro VL (2020) Iodinated metallacrowns: toward combined bimodal near-infrared and X-ray contrast imaging agents. Chem Eur J 26:1274–1277
Bruker Apex3 and SAINT; Bruker AXS Inc.: Madison, WI
Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D (2015) Comparison of silver and molybdenum Microfocus X-ray sources for single-crystal structure determination. J Appl Cryst 48:3–10
SHELXTL suite of programs, Version 6.14, 2000–2003, Bruker Advanced X-ray Solutions, Bruker AXS Inc., Madison, Wisconsin: USA
Sheldrick GM (2008a) A short history of SHELX. Acta Cryst A64:112–122
Sheldrick GM (2018) SHELXL2018. University of Göttingen, Germany
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8
Hübschle CB, Sheldrick GM, Dittrich B (2011) ShelXle: a Qt Graphical User Interface for SHELXL. J Appl Cryst 44:1281–1284
Sheldrick GM (2008b) CELL_NOW. University of Göttingen, Germany
Sheldrick GM (2012) TWINABS. University of Göttingen, Germany
Wang J, Lu G, Liu Y, Wu SG, Huang GZ, Liu JL, Tong ML (2019) Building block and directional bonding approaches for the synthesis of {DyMn4}n (n = 2, 3) metallacrown assemblies. Cryst Growth Des 19:1896–1902
Liu W, Thorp HH (1993) Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. 2. Refined distances and other enzymes. Inorg Chem 32:4102–4105
Trzesowska A, Kruszynski R, Bartczak TJ (2004) New bond-valence parameters for lanthanides. Acta Cryst B60:174–178
Llunell M, Casanova D, Cirera J, Alemany P, Alvarez S (2013) SHAPE, version 2.1; Barcelona, Spain
Pinksy M, Avnir D (1998) Continuous symmetry measures. 5.The Classical. Polyhedra Inorg Chem 37:5575–5582
Casanova D, Cirera J, Llunell M, Alemany P, Avnir D, Alvarez S (2004) Minimal distortion pathways in polyhedral rearrangements. J Am Chem Soc 126:1755–1763
Cirera J, Ruiz E, Alvarez S (2005) Continuous shape measures as a stereochemical tool in organometallic chemistry. Organometallics 24:1556–1562
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van de Streek J (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39:453–457
Funding
This work was funded by the PASSHE Faculty Professional Development Council Grant Program, the Shippensburg University (SU) Faculty Professional Development Council Grant Program, the SU Student/Faculty Research Engagement Grant, and the SU and Shippensburg Foundation Undergraduate Research Program to CMZ. The Bruker AXS D8 Quest CMOS X-ray diffractometers were funded by the National Science Foundation through the Major Research Instrumentation Program under Grant No. CHE 1625543 to MZ.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Collin M. Foley, Maikel A. Armanious, Alyssa M. Smihosky, Matthias Zeller, and Curtis M. Zaleski. The first draft of the manuscript was written by Curtis M. Zaleski and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Availability of Data and Material
CCDC 2030723-2030737 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Foley, C.M., Armanious, M.A., Smihosky, A.M. et al. Syntheses and Crystal Structures of a Series of Manganese-Lanthanide-Sodium 12-Metallacrown-4 Dimers. J Chem Crystallogr 51, 465–482 (2021). https://doi.org/10.1007/s10870-020-00870-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-020-00870-1