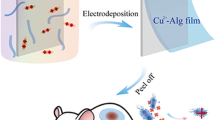
Abstract
Wound dressings play important roles in the management of wounds, and calcium cross-linked alginate (Ca2+-Alg) is a commonly used hydrogel that is adapted for wound treatment. However, conventional methods for fabricating Ca2+-Alg hydrogels can be tedious and difficult to control because of the rapid Ca2+-induced gelation of alginate. In this study, An electrodeposition method was used to rapidly and controllably fabricate Ca2+-Alg films for wound treatment. Several measures of film growth (e.g., thickness and mass) are shown to linearly correlate to the imposed charge transfer at the electrode. Similarly, this charge transfer was also observed to control important physicochemical wound healing properties such as water uptake and retention capacity. Furthermore, a wound healing animal test was performed to evaluate the performance of this electro-fabricated calcium alginate film for wound treatment. This in vivo study demonstrated that wounds dressed with an electro-fabricated Ca2+-Alg film closed faster than that of untreated wounds. Further, the new dermis tissue that formed was composed of reorganized and stratified epithelial layer, with fully developed connective tissue, hair follicle, sebaceous glands as well as aligned collagen. Therefore, our study indicates that this electrofabrication method for the rapid and controlled preparation of alginate film could provide exciting opportunities for wound treatment. More broadly, this study demonstrates the potential of electrochemistry for the fabrication of high performance polymeric materials.
Graphical Abstract
Here we report a rapid and controllable fabrication of free-standing alginate films by coupling anodic electrodeposition with subsequent peeling of deposited materials for wound dressing.







Similar content being viewed by others
References
Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing-a review[J]. J Pharm Sci. 2015;104(11):3653–80.
Reyes-Ortega F, Cifuentes A, Rodriguez G, et al. Bioactive bilayered dressing for compromised epidermal tissue regeneration with sequential activity of complementary agents[J]. Acta Biomater. 2015;23:103–5.
Li J, Wu Y, He J, et al. A new insight to the effect of calcium concentration on gelation process and physical properties of alginate films[J]. J Mater Sci Mater Med. 2016;51(12):5791–801.
Xu R, Luo G, Xia H, et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction[J]. Biomaterials. 2015;40:1–11.
Jayakumar R, Prabaharan M, Kumar PTS, et al. Biomaterials based on chitin and chitosan in wound dressing applications[J]. Biotechnol Adv. 2011;29(3):322–37.
Bruchet M, Melman A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange[J]. Carbohydr Polym. 2015;131:57–64.
Tallis EE. The Structure of Alginate Fibres[J]. J Text Inst Trans. 1950;41:T151–T58.
Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials[J]. Macromol Biosci. 2006;6(8):623–33.
Braccini I, Perez S. Molecular basis of Ca2+-induced gelation in alginates and pectins: the egg-box model revisited[J]. Biomacromolecules. 2001;2(4):1089–96.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications[J]. Prog Polym Sci. 2012;37(1):106–26.
Sayag J, Meaume S, Bohbot S. Healing properties of calcium alginate dressings[J]. J Wound Care. 1996;8:357–62.
Alexander BR, Murphy KE, Gallagher J, et al. Gelation time, homogeneity, and rupture testing of alginate-calcium carbonate-hydrogen peroxide gels for use as wound dressings[J]. J Biomed Mater Res B Appl Biomater. 2012;100B(2):425–31.
Pankongadisak P, Ruktanonchai UR, Supaphol P, et al. Development of silver nanoparticles-loaded calcium alginate beads embedded in gelatin scaffolds for use as wound dressings[J]. Polym Int. 2015;64(2):275–83.
Qin Y. Alginate fibres: an overview of the production processes and applications in wound management[J]. Polym Int. 2008;57(2):171–180.
Esser E, Tessmar JKV. Preparation of well-defined calcium cross-linked alginate films for the prevention of surgical adhesions †[J]. J Biomed Mater Res B Appl Biomater. 2013;101B(5):826–39.
Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties[J]. Biomaterials. 2001;22(6):511–21.
Skj G. Inhomogeneous polysaccharide ionic gels[J]. Carbohydr Polym. 1989;10(1):31–54.
Chueh BH, Zheng Y, Torisawa YS, et al. Patterning alginate hydrogels using light-directed release of caged calcium in a microfluidic device[J]. Biomed Microdevices. 2010;12(1):145–51.
Liu K, Ding HJ, Liu J, et al. Shape-controlled production of biodegradable calcium alginate gel microparticles using a novel microfluidic device[J]. Langmuir. 2006;22(22):9453–57.
Rydzek G, Terentyeva TG, Pakdel A, et al. Simultaneous electropolymerization and electro-click functionalization for highly versatile surface platforms[J]. ACS Nano. 2014;8(5):5240–8.
Peng X, Liu Y, Bentley WE et al. Electrochemical fabrication of functional gelatin-based bio-electronic interface[J]. Biomacromolecules. 2016;17(2):558–63.
Ehret F, Wu H, Alexander S, et al. Electrochemical control of rapid bioorthogonal tetrazine ligations for selective functionalization of microelectrodes[J]. J Am Chem Soc. 2015;137(28):8876.
Xie C, Lu X, Wang K, et al. Pulse electrochemical driven rapid layer-by-layer assembly of polydopamine and hydroxyapatite nanofilms via alternative redox in situ synthesis for bone regeneration[J]. ACS Biomater Sci Eng. 2016;2(6):920–8.
Li G, Cao H, Zhang W, et al. Enhanced osseointegration of hierarchical micro/nano-topographic titanium fabricated by micro-arc oxidation and electrochemical treatment[J]. ACS Appl Mater Interfaces. 2016;8(6):3840.
Cheong M, Zhitomirsky I. Electrodeposition of alginic acid and composite films[J]. Physicochem Eng Asp. 2008;328(1-3):73–8.
Cheng Y, Luo X, Betz J, et al. Mechanism of anodic electrodeposition of calcium alginate[J]. Soft Matter. 2011;7(12):5677–84.
Shi XW, Tsao CY, Yang XH, et al. Electroaddressing of cell populations by co-deposition with calcium alginate hydrogels[J]. Adv Funct Mater. 2009;19(13):2074–2080.
Yang XH, Kim E, Liu Y, et al. In-film bioprocessing and immunoanalysis with electroaddressable stimuli-responsive polysaccharides[J]. Adv Funct Mater. 2010;20(10):1645–52.
Minton TK, Wright ME, Tomczak SJ, et al. Atomic oxygen effects on poss polyimides in low earth orbit[J]. ACS Appl Mater Interfaces. 2012;4(2):492–502.
Hurteaux R, Benhayoune H, Edwards-Levy F, et al. Preparation and characterization of an electrodeposited calcium phosphate coating associated with a calcium alginate matrix[J]. J Mater Sci Mater Med. 2005;16(1):9–13.
Liu Y, Zhang B, Gray KM, et al. Electrodeposition of a weak polyelectrolyte hydrogel: remarkable effects of salt on kinetics, structure and properties[J]. Soft Matter. 2013;9(9):2703.
Dai GL, Wan WF, Zhao YL, et al. Controllable 3D alginate hydrogel patterning via visible-light induced electrodeposition[J]. Biofabrication. 2016;8(2):025004.
Wan WF, Dai GL, Zhang LJ, et al. Paper-based electrodeposition chip for 3D alginate hydrogel formation[J]. Micromachines. 2015;6(10):1546–59.
Sriamornsak P, Kennedy RA. A novel gel formation method, microstructure and mechanical properties of calcium polysaccharide gel films[J]. Int J Pharm. 2006;323(1-2):72–80.
Ben Amara C, Eghbal N, Oulahal N, et al. Properties of lysozyme/sodium alginate complexes for the development of antimicrobial films[J]. Food Res Int. 2016;89(pt1):272.
Zhang C, Xu Q, Xiong C, et al. Properties and structure of calcium alginate sponge[J]. Polym Mater Sci Eng. 2012;28(9):24–7.
Jayash SN, Hashim NM, Misran M, et al. Formulation and in vitro and in vivo evaluation of a new osteoprotegerin-chitosan gel for bone tissue regeneration[J]. J Biomed Mater Res A. 2017;105(2):398–407.
Wang X, Ge J, Tredget EE, et al. The mouse excisional wound splinting model, including applications for stem cell transplantation[J]. Nat Protoc. 2013;8(2):302–9.
Lowe A, Bills J, Verma R, et al. Electrospun nitric oxide releasing bandage with enhanced wound healing[J]. Acta Biomater. 2015;13:121–30.
Fan Z, Liu B, Wang J, et al. A novel wound dressing based on Ag/graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing[J]. Adv Funct Mater. 2014;24(25):3933–43.
Jaiswal M, Koul V, Dinda AK. In vitro and in vivo investigational studies of a nanocomposite-hydrogel-based dressing with asilver-coated chitosan wafer for full-thickness skin wounds[J]. J Appl Polym Sci. 2016;133(21).
Qin Y. The gel swelling properties of alginate fibers and their applications in wound management[J]. Polym Adv Technol. 2008;19(1):6–14.
Straccia MC, D’ayala GG, Romano I, et al. Alginate hydrogels coated with chitosan for wound dressing[J]. Mar Drugs. 2015;13(5):2890–908.
Sun L, Huang Y, Bian Z, et al. Sundew-inspired adhesive hydrogels combined with adipose-derived stem cells for wound healing[J]. ACS Appl Mater Interfaces. 2016;8(3):2423–34.
Chandika P, Ko SC, Jung WK. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration[J]. Int J Biol Macromol. 2015;77:24–35.
Lansdown AB. Calcium: a potential central regulator in wound healing in the skin[J]. Wound Repair & Regen. 2002;10(5):271–85.
Acknowledgements
The support from the National Natural Science Foundation of China (51103043, 51573047), the 111 project (B14018), the Fundamental Research Funds for the Central Universities (222201717002) and the United States National Science Foundation (CBET-1435957) and Defense Threat Reduction Agency (HDTRA1-13-0037).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Liu, X., Liu, H., Qu, X. et al. Electrical signals triggered controllable formation of calcium-alginate film for wound treatment. J Mater Sci: Mater Med 28, 146 (2017). https://doi.org/10.1007/s10856-017-5956-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5956-x