Abstract
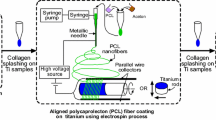
The surface of medical implant alloy Ti-6Al-4V was chemically modified to allow it to covalently bond with collagen/PVA nanofibers. These nanofibers were successfully attached to the Ti-6Al-4V surface in three different morphologies: randomly oriented high-density fiber, COL(H); randomly oriented low-density fiber, COL(L); and aligned high-density fiber, COL(A). The effects of the morphology of these covalently-bound collagen nanofibers on the growth and differentiation of osteoblasts were studied for 21 days. The low-density nanofibers covered approximately 80% of the Ti64 surface, while the high-density nanofibers covered nearly 100%. These covalently attached fibrous coatings remained attached to the metal surface after 3 weeks of cell culture. In the first week the aligned fibers of COL(A) allowed the osteoblasts to stretch and elongate in the direction of the fibers. This directional elongation was not seen in the cells on the randomly-oriented samples. Cells proliferated and differentiated on all three surfaces over time. By the end of the test, the amount of type I collagen secreted by the cells on COL(H) was the highest, while the degree of mineralization was highest on COL(A) among the three samples (p < 0.05). Different nanofiber morphologies changed the cell morphology and the secretion of cellular products. The mechanisms remained to be investigated.
Graphical abstract
The surface of medical implant alloy Ti-6Al-4V was chemically modified to allow it to covalently bond with collagen/PVA nanofibers. The SEM micrographs in the top row show the random and aligned morphology of the collagen-PVA nanofibers. The nanofibers on COL(A) were aligned in the general direction indicated by the arrow. The second row are images from EDX titanium element mapping. The location of the titanium elements are shown as bright dots. The low-density nanofibers, COL(L), covered approximately 80% of the Ti64 surface, while the high-density nanofibers, COL(H) and COL(A), covered nearly 100%.
All three surfaces demonstrated good biocompatibility for the cultured osteoblasts. The fiber alignment seemed to have an effect on early cellular morphology (day 7), collagen secretion and calcium deposition, while the density of the fibers seemed to have no significant effect on cell behavior.
SEM micrographs of osteoblasts after 7 and 14 days of cell culture are shown in the third and fourth rows. The surface of COL(L) has more cell-free spots indicated by (*) on day 7 as other two surfaces were covered by cells. The nanofibers could no longer be observed and were covered with mineralized granules (circles) after 14 days of cell culture. The cells appear stretched out on the mineralized granules.




Similar content being viewed by others
References
Hidalgo-Bastida LA, Cartmell SH. Mesenchymal stem cells, osteoblasts and extracellular matrix proteins: enhancing cell adhesion and differentiation for bone tissue engineering. Tissue Eng Part B Rev. 2010;16(4):405–12.
Munisamy S, Vaidyanathan TK, Vaidyanathan J. A bone-like precoating strategy for implants: collagen immobilization and mineralization on pure titanium implant surface. J Oral Implantol. 2008;34(2):67–75.
Mizuno M, Kuboki Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J Biochem. 2001;129(1):133–8.
Keogh MB, O’ Brien FJ, Daly JS. A novel collagen scaffold supports human osteogenesis–applications for bone tissue engineering. Cell Tissue Res. 2010;340(1):169–77.
Chevallay B, Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med Biol Eng Comput. 2000;38(2):211–8.
Roehlecke C, Witt M, Kasper M, Schulze E, Wolf C, Hofer A, et al. Synergistic effect of titanium alloy and collagen type I on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs. 2001;168(3):178–87.
Zhao N, Workman B, Zhu DH. Endothelialization of novel magnesium-rare earth alloys with fluoride and collagen coating. Int J Mol Sci. 2014;15(4):5263–76.
Becker D, Geissler U, Hempel U, Bierbaum S, Scharnweber D, Worch H, et al. Proliferation and differentiation of rat calvarial osteoblasts on type I collagen-coated titanium alloy. J Biomed Mater Res. 2002;59(3):516–27.
Masi L, Franchi A, Santucci M, Danielli D, Arganini L, Giannone V, et al. Adhesion, growth, and matrix production by osteoblasts on collagen substrata. Calcif Tissue Int. 1992;51(3):202–12.
Andrianarivo AG, Robinson JA, Mann KG, Tracy RP. Growth on type I collagen promotes expression of the osteoblastic phenotype in human osteosarcoma MG-63 cells. J Cell Physiol. 1992;153(2):256–65.
Lynch MP, Stein JL, Stein GS, Lian JB. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp Cell Res. 1995;216(1):35–45.
Ong JL, Bess EG, Bessho K. Osteoblast progenitor cell responses to characterized titanium surfaces in the presence of bone morphogenetic protein-atelopeptide type I collagen in vitro. J Oral Implantol. 1999;25(2):95–100.
Alekseeva T, Hadjipanayi E, Abou Neel EA, Brown RA. Engineering stable topography in dense bio-mimetic 3D collagen scaffolds. Eur Cell Mater. 2012;23:28–40.
Ber S, Torun Kose G, Hasirci V. Bone tissue engineering on patterned collagen films: an in vitro study. Biomaterials. 2005;26(14):1977–86.
Landis WJ, Song MJ, Leith A, McEwen L, McEwen BF. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J Struct Biol. 1993;110(1):39–54.
Su XW, Feng QL, Cui FZ, Zhu XD. Microstructure and micromechanical properties of the mid-diaphyses of human fetal femurs. Connect Tissue Res. 1997;36(3):271–86.
Traub W, Arad T, Weiner S. Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc Natl Acad Sci U S A. 1989;86(24):9822–6.
Zhang J, Senger B, Vautier D, Picart C, Schaaf P, Voegel JC, et al. Natural polyelectrolyte films based on layer-by layer deposition of collagen and hyaluronic acid. Biomaterials. 2005;26(16):3353–61.
Lin HY, Kuo YJ, Chang SH, Ni TS. Characterization of electrospun nanofiber matrices made of collagen blends as potential skin substitutes. Biomed Mater. 2013;8(2):025009.
Fiorani A, Gualandi C, Panseri S, Montesi M, Marcacci M, Focarete ML, et al. Comparative performance of collagen nanofibers electrospun from different solvents and stabilized by different crosslinkers. J Mater Sci Mater Med. 2014;25(10):2313–21.
Kazanci M. Solvent and temperature effects on folding of electrospun collagen nanofibers. Mater Lett. 2014;130:223–6.
Bumgardner JD, Wiser R, Elder SH, Jouett R, Yang Y, Ong JL. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J Biomater Sci Polym Ed. 2003;14(12):1401–9.
Bumgardner JD, Wiser R, Gerard PD, Bergin P, Chestnutt B, Marin M, et al. Chitosan: potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J Biomater Sci Polym Ed. 2003;14(5):423–38.
Chen ZG, Wang PW, Wei B, Mo XM, Cui FZ. Electrospun collagen-chitosan nanofiber: A biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010;6(2):372–82.
Khang D, Sato M, Price RL, Ribbe AE, Webster TJ. Selective adhesion and mineral deposition by osteoblasts on carbon nanofiber patterns. Int J Nanomedicine. 2006;1(1):65–72.
Barrere F, van Blitterswijk CA, de Groot K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int J Nanomedicine. 2006;1(3):317–32.
Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63(15):2223–53.
El-Said WA, Yea CH, Jung M, Kim H, Choi JW. Analysis of effect of nanoporous alumina substrate coated with polypyrrole nanowire on cell morphology based on AFM topography. Ultramicroscopy. 2010;110(6):676–81.
Raimondo T, Puckett S, Webster TJ. Greater osteoblast and endothelial cell adhesion on nanostructured polyethylene and titanium. Int J Nanomedicine. 2010;5:647–52.
Meng ZX, Wang YS, Ma C, Zheng W, Li L, Zheng YF. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mat Sci Eng C-Mater. 2010;30(8):1204–10.
Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9.
Gu Q, Zhu HM, Zhang XJ. Apoptosis of rat osteoblasts in process of calcification in vitro. Acta Pharmacol Sin. 2002;23(9):808–12.
Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992;7(6):683–92.
Taylor SE, Shah M, Orriss IR. Generation of rodent and human osteoblasts. Bonekey Rep. 2014;3:585.
Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235(1):176–90.
Heinemann C, Heinemann S, Bernhardt A, Worch H, Hanke T. Novel textile chitosan scaffolds promote spreading, proliferation, and differentiation of osteoblasts. Biomacromolecules. 2008;9(10):2913–20.
Frohbergh ME, Katsman A, Botta GP, Lazarovici P, Schauer CL, Wegst UG, et al. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials. 2012;33(36):9167–78.
McEwen BF, Song MJ, Landis WJ. Quantitative determination of the mineral distribution in different collagen zones of calcifying tendon using high voltage electron microscopic tomography. J Comput Assist Microsc. 1991;3(4):201–10.
Su X, Sun K, Cui FZ, Landis WJ. Organization of apatite crystals in human woven bone. Bone. 2003;32(2):150–62.
Acknowledgements
This research is sponsored by Ministry of Science and Technology, Taiwan (MOST 104-2221-E-027-101 and NSC 101-2221-E-027-140) and National Taipei University of Technology and MacKay Memorial Hospital Joint Research Program (NTUT-MMH-104-02)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Lin, HY., Peng, ZX. Nanofibers grafted on titanium alloy: the effects of fiber alignment and density on osteoblast mineralization. J Mater Sci: Mater Med 28, 140 (2017). https://doi.org/10.1007/s10856-017-5951-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5951-2