Abstract
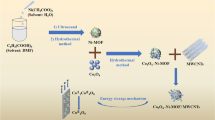
Many studies have been done to control the electrochemical performance of electrodes for use in supercapacitors. Depending on the morphology, size, and structure of the material used in electrode design, the fabricated electrode exhibits supercapacitor or battery-like behavior. In this work, we report the easy electrochemical synthesis of Ni(OH)2/MWCNT onto Anodize Commercial Graphite (ACG) sheet electrode and the effect deposition time of Ni(OH)2 on the supercapacitor behavior of the electrode. Ni(OH)2/MWCNT/ACG sheet electrode was easily fabricated through electrochemical anodization of commercial graphite sheet followed by electrodeposition of MWCNT and Ni(OH)2 at different times. The effect of deposition time of Ni(OH)2 as well as the effect of anodization of commercial graphite sheet on supercapacitive behavior of the constructed electrode was investigated and proved by surface morphology (FE-SEM), CV, GCD, and EIS electrochemical tests at 1.0 M NaOH solution. Also, XRD and EDX analyses confirmed that MWCNT and Ni(OH)2 were deposited on the electrode. To investigate the operational use of the fabricated electrode, a symmetric solid-state supercapacitor device was constructed with the optimized electrode, and its electrochemical performance was investigated. The results showed that the optimized supercapacitor device has a good capacitance of 115 mF cm−2 at 1 mA cm−2 and cyclic life of 72.18% after 8000 GCD cycles.









Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
S. Banerjee, B. De, P. Sinha, et al., Applications of supercapacitors. in Handbook of Nanocomposite Supercapacitor Materials I. (Springer, New York, 2020), pp. 341–350
A. González, E. Goikolea, J.A. Barrena, R. Mysyk, Review on supercapacitors: technologies and materials. Renew. Sustain. Energy Rev. 58, 1189–1206 (2016)
K. Kim, J. Park, J. Lee, et al., Ultrafast PEDOT: PSS/H2SO4 electrical double layer capacitors: comparison with pani pseudocapacitors. ChemSusChem (2022)
A.G. Pandolfo, A.F. Hollenkamp, Carbon properties and their role in supercapacitors. J. Power. Sources 157, 11–27 (2006)
E. Frackowiak, Electrode materials with pseudocapacitive properties. Supercapacitors Mater. Syst. Appl. 207–237 (2013)
D. Sarkar, G.G. Khan, A.K. Singh, K. Mandal, High-performance pseudocapacitor electrodes based on α-Fe2O3/MnO2 core–shell nanowire heterostructure arrays. J. Phys. Chem. C 117, 15523–15531 (2013)
S. Kumar, G. Saeed, L. Zhu et al., 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: a review. Chem. Eng. J. 403, 126352 (2021)
S. Natarajan, M. Ulaganathan, V. Aravindan, Building next-generation supercapacitors with battery type Ni (OH)2. J. Mater. Chem. A 9, 15542–15585 (2021)
Y. Luo, Y. Li, D. Wang et al., Hierarchical α-Ni (OH)2 grown on CNTs as a promising supercapacitor electrode. J. Alloys Compd. 743, 1–10 (2018)
S. Asaithambi, P. Rajkumar, A.S. Rasappan et al., Designed nanoarchitectonics and fabrication of Ni (OH)2/MWCNT/CNF electrode for asymmetric hybrid supercapacitor applications. J Energy Storage 72, 108532 (2023)
J. Yu, B. Wang, Q. Lu et al., Cathode glow discharge electrolysis synthesis of flower-like β-Ni (OH)2 microsphere for high-performance supercapacitor. Chem. Eng. J. 453, 139769 (2023)
S. Min, C. Zhao, Z. Zhang et al., Synthesis of Ni (OH)2/RGO pseudocomposite on nickel foam for supercapacitors with superior performance. J. Mater. Chem. A 3, 3641–3650 (2015)
B.K. Kim, V. Chabot, A. Yu, Carbon nanomaterials supported Ni (OH)2/NiO hybrid flower structure for supercapacitor. Electrochim. Acta 109, 370–380 (2013)
F. Gobal, M. Faraji, Fabrication of nanoporous nickel oxide by de-zincification of Zn–Ni/(TiO2-nanotubes) for use in electrochemical supercapacitors. Electrochim. Acta 100, 133–139 (2013)
D. Mandal, P. Routh, A.K. Mahato, A.K. Nandi, Electrochemically modified graphite paper as an advanced electrode substrate for supercapacitor application. J. Mater. Chem. A 7, 17547–17560 (2019)
R. Dadashi, M. Bahram, M. Faraji, Fabrication of a solid-state symmetrical supercapacitor based on polyaniline grafted multiwalled carbon nanotube deposit onto created vertically oriented graphene nanosheets on graphite sheet. J. Energy Storage 52, 104775 (2022)
L. Fagiolari, M. Sampò, A. Lamberti et al., Integrated energy conversion and storage devices: interfacing solar cells, batteries and supercapacitors. Energy Storage Mater. 51, 400–434 (2022)
Y. Wang, Y. Xia, Recent progress in supercapacitors: from materials design to system construction. Adv. Mater. 25, 5336–5342 (2013)
P. Balaya, Size effects and nanostructured materials for energy applications. Energy Environ. Sci. 1, 645–654 (2008)
J. Duay, S.A. Sherrill, Z. Gui et al., Self-limiting electrodeposition of hierarchical MnO2 and M (OH)2/MnO2 nanofibril/nanowires: mechanism and supercapacitor properties. ACS Nano 7, 1200–1214 (2013)
M.S. Javed, A. Mateen, I. Hussain et al., Recent progress in the design of advanced MXene/metal oxides-hybrid materials for energy storage devices. Energy Storage Mater. 53, 827–872 (2022)
D.P. Dubal, O. Ayyad, V. Ruiz, P. Gomez-Romero, Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 44, 1777–1790 (2015)
H. Liu, X. Liu, S. Wang et al., Transition metal based battery-type electrodes in hybrid supercapacitors: a review. Energy Storage Mater. 28, 122–145 (2020)
C. Liu, F. Li, L. Ma, H. Cheng, Advanced materials for energy storage. Adv. Mater. 22, E28–E62 (2010)
J. Liu, J. Wang, C. Xu et al., Advanced energy storage devices: basic principles, analytical methods, and rational materials design. Adv. Sci. 5, 1700322 (2018)
S.K. Meher, P. Justin, G.R. Rao, Nanoscale morphology dependent pseudocapacitance of NiO: influence of intercalating anions during synthesis. Nanoscale 3, 683–692 (2011)
R.S. Kate, S.A. Khalate, R.J. Deokate, Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: a review. J. Alloys Compd. 734, 89–111 (2018)
B. Babakhani, D.G. Ivey, Effect of electrodeposition conditions on the electrochemical capacitive behavior of synthesized manganese oxide electrodes. J. Power. Sources 196, 10762–10774 (2011)
H. Gong, S.-T. Kim, J.D. Lee, S. Yim, Simple quantification of surface carboxylic acids on chemically oxidized multi-walled carbon nanotubes. Appl. Surf. Sci. 266, 219–224 (2013)
G.R. Fu, Z.A. Hu, L. Xie et al., Electrodeposition of nickel hydroxide films on nickel foil and its electrochemical performances for supercapacitor. Int. J. Electrochem. Sci. 4, 1052 (2009)
S. Parveen, S.K. Sharma, S.N. Pandey, Solid-state symmetric supercapacitor based on Y doped Sr (OH)2 using SILAR method. Energy 197, 117163 (2020)
W. Mahfoz, S.S. Shah, M.A. Aziz, A.-R. Al-Betar, Fabrication of high-performance supercapacitor using date leaves-derived submicron/nanocarbon. J. Saudi Chem. Soc. 26, 101570 (2022)
M.A. Yewale, R.A. Kadam, N.K. Kaushik et al., Interconnected plate-like NiCo2O4 microstructures for supercapacitor application. Mater. Sci. Eng. B 287, 116072 (2023)
N. Liu, Z. Peng, Y. Tian et al., Cobalt pentlandite structured (Fe Co, Ni) 9S8: fundamental insight and evaluation of hybrid supercapacitor. Appl. Surf. Sci. 611, 155568 (2023)
B.I. Rosario-Castro, E.J. Contés, M. Lebrón-Colón et al., Combined electron microscopy and spectroscopy characterization of as-received, acid purified, and oxidized HiPCO single-wall carbon nanotubes. Mater. Charact. 60, 1442–1453 (2009)
B. Jansi Rani, N. Dhivya, G. Ravi et al., Electrochemical performance of β-Nis@ Ni(OH)2 nanocomposite for water splitting applications. ACS Omega 4, 10302–10310 (2019)
X. Ni, Q. Zhao, B. Li et al., Interconnected β-Ni(OH)2 sheets and their morphologay-retained transformation into mesostructured Ni. Solid State Commun. 137, 585–588 (2006)
G.J. de Soler-Illia, M. Jobbágy, A.E. Regazzoni, M.A. Blesa, Synthesis of nickel hydroxide by homogeneous alkalinization. Precipitation mechanism. Chem. Mater. 11, 3140–3146 (1999)
P. Nie, C. Min, H.-J. Song et al., Preparation and tribological properties of polyimide/carboxyl-functionalized multi-walled carbon nanotube nanocomposite films under seawater lubrication. Tribol. Lett. 58, 1–12 (2015)
J. Tientong, S. Garcia, C.R. Thurber,T.D. Golden, Synthesis of nickel and nickel hydroxide nanopowders by simplified chemical reduction. J. Nanotechnol. (2014)
L. Wang, X. Li, T. Guo et al., Three-dimensional Ni(OH)2 nanoflakes/graphene/nickel foam electrode with high rate capability for supercapacitor applications. Int. J. Hydrog. Energy 39, 7876–7884 (2014)
S. Sun, P. Diao, C. Feng et al., Nickel-foam-supported β-Ni (OH)2 as a green anodic catalyst for energy efficient electrooxidative degradation of azo-dye wastewater. RSC Adv. 8, 19776–19785 (2018)
D.A. Corrigan, R.M. Bendert, Effect of coprecipitated metal ions on the electrochemistry of nickel hydroxide thin films: cyclic voltammetry in 1M KOH. J. Electrochem. Soc. 136, 723 (1989)
P.J. Hall, M. Mirzaeian, S.I. Fletcher et al., Energy storage in electrochemical capacitors: designing functional materials to improve performance. Energy Environ. Sci. 3, 1238–1251 (2010)
V. Augustyn, P. Simon, B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014)
K.J. Aoki, J. Chen, Y. Liu, B. Jia, Peak potential shift of fast cyclic voltammograms owing to capacitance of redox reactions. J. Electroanal. Chem. 856, 113609 (2020)
A.T.A. Ahmed, H.S. Chavan, Y. Jo et al., One-step facile route to copper cobalt sulfide electrodes for supercapacitors with high-rate long-cycle life performance. J. Alloys Compd. 724, 744–751 (2017)
A.C. Lokhande, A. Patil, A. Shelke et al., Binder-free novel Cu4SnS4 electrode for high-performance supercapacitors. Electrochim. Acta 284, 80–88 (2018)
M. Sun, J. Tie, G. Cheng et al., In situ growth of burl-like nickel cobalt sulfide on carbon fibers as high-performance supercapacitors. J. Mater. Chem. A 3, 1730–1736 (2015)
K.M. Thulasi, S.T. Manikkoth, A. Paravannoor et al., Supercapacitor electrodes based on modified titania nanotube arrays on flexible substrates. Int. J. Mater. Res. 112, 937–944 (2021)
R. Dadashi, M. Bahram, M. Faraji, Polyaniline-tungsten oxide nanocomposite co-electrodeposited onto anodized graphene oxide nanosheets/graphite electrode for high performance supercapacitor device. J. Appl. Electrochem. 53(5), 893–908 (2023)
R. Dadashi, M. Bahram, K. Farhadi, In-situ growth of Cu nanoparticles-polybenzidine over GO sheets onto graphite sheet as a novel electrode material for fabrication of supercapacitor device. J Energy Storage 79, 110038 (2024)
P. Zoltowski, On the electrical capacitance of interfaces exhibiting constant phase element behaviour. J. Electroanal. Chem. 443, 149–154 (1998)
T.C. Girija, M.V. Sangaranarayanan, Analysis of polyaniline-based nickel electrodes for electrochemical supercapacitors. J. Power. Sources 156, 705–711 (2006)
S.-K. Hwang, S.J. Patil, N.R. Chodankar et al., An aqueous high-performance hybrid supercapacitor with MXene and polyoxometalates electrodes. Chem. Eng. J. 427, 131854 (2022)
Y.A. Tarek, R. Shakil, A.H. Reaz et al., Wrinkled Flower-Like rGO intercalated with Ni(OH)2 and MnO2 as high-performing supercapacitor electrode. ACS Omega 7, 20145–20154 (2022)
V.M. Poonam, D.K. Jangid et al., Investigation of supercapacitor cyclic degradation through impedance spectroscopy and Randles circuit model. Energy Storage 4, e355 (2022)
Acknowledgements
The authors wish to express thanks to the office of the vice chancellor of research of Urmia University—Iran for the financial support.
Funding
This work was supported by the Research Affairs of Urmia University.
Author information
Authors and Affiliations
Contributions
RD; methodology, investigation, data curation, writing. MB; investigation, writing—review and editing, Supervisor. MF; investigation, writing—review and editing, Supervisor. All the authors were involved in the preparation of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dadashi, R., Bahram, M. & Faraji, M. Fabrication of symmetric solid-state Ni(OH)2/MWCNT/ACG supercapacitor and more investigation of surface morphology on its capacitive behavior. J Mater Sci: Mater Electron 35, 852 (2024). https://doi.org/10.1007/s10854-024-12593-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12593-6