Abstract
The influences of annealing temperature (473–573 K) on the crystal structure, linear/nonlinear optical parameters, and electrical characteristics of 80 nm thick indium-doped tin oxide (ITO) thin films are investigated. Thermal annealing induces the crystal structure in the ITO. As-prepared and annealed ITO have various morphologies depending on the annealing temperature, such as nanoplates and dendritic and spherical nanoparticles. As the substrate temperature increased up to 370 K, the electrical resistivity and sheet resistance of as-prepared ITO decreased dramatically and then slightly decreased as the substrate temperature further increased. The electrical conductivity and activation energy for the various processes were estimated. The reflectance (R) and transmittance (T) data are used to calculate the linear/nonlinear optical constants and parameters. The optical bandgap increased from 3.18 to 3.8 eV as the annealing temperature increased from room temperature to 573 K. Crystallinity is improved due to the annealing and hence an enhancement in the optical energy bandgap is achieved. Meanwhile, high-temperature annealing reversibly affected the optical bandgap energy of ITO thin films via reduction and oxidation reactions. Thermal annealing of ITO films improves crystal structure, visual transparency, and electrical conductivity, making it the preferred material for optoelectronic devices and solar cells.
Similar content being viewed by others
1 Introduction
Indium-doped tin oxide (ITO) thin films remain popular due to their numerous technical applications in sensing, solar cells, heat-reflecting mirrors, and energy [1, 2]. The ITO films are n-type semiconductors with a wide bandgap (∼ 3.5–4.3 eV) and low electrical resistivity with a high value of transparency and reflectance in the visible and infrared regions [3]. It has excellent optoelectronic properties due to its transparency (85–90%) in the visible region and very low electrical resistivity at room temperature [4]. Consequently, ITO can be employed in optoelectronic technology, among other things. A broad bandgap semiconductor is the most advantageous host compound for diluting magnetic semiconductors with high Curie temperature [5].
Doping and annealing processes can be used to control the properties of ITO [6]. Typically, annealing is used to increase the crystallinity of substances or shift their crystal structure from an amorphous to a crystalline state. For example, increasing the temperature from ambient temperature to 200 °C leads amorphous ITO to change to polycrystalline material, and increasing the optical gap energies [7]. Further rise in the temperature reduces the optical bandgap energies of ITO films, owing to electron–phonon interactions. However, reversible fluctuations in optical gap energies were observed based on the background partial pressure of oxygen, indicating that the reduction/oxidation processes proceed aggressively according to the ITO thin films' low reduction/oxidation potential energies at high temperatures [8].
The ITO film is the most prominent transparent conducting oxide and industry standard material. Moreover, the ITO film's high optical transparency and magnificent electrical conductivity make it suitable for optoelectronic applications for instant touch screens/panel contact, electrochromic displays, electrodes for LCD, gas sensing, and antifogging airplane windows [1, 9]. Various ITO powder types with small dimensions, structure, and surface area have been developed for application in electrical devices [10]. In contrast, the ITO nanocrystalline material's stable cubic bixbyite phases are only related to high electrical conductivity [11]. The electrical behavior of ITO nanocrystalline materials, particularly at low temperatures, and the insulator to metal transitions broaden the scope for further investigation into the low-temperature behavior of these materials [12].
Few studies have investigated nanocrystalline powder and bulk usage of ITO films in optoelectronic applications [13] and the effect of deposition temperature (150–180 °C) and annealing on crystalline structural changes [14]. According to the study, annealing temperatures over 250 °C are required to transform amorphous ITO into polycrystalline ITO. Typically, ITO is deposited using the evaporation, radio frequency (RF) sputtering, and sol–gel techniques [15, 16]. RF sputtering is used to generate ITO films due to low-temperature metallization techniques, and suggestions for conductive oxide layer metallization are being established [17, 18].
The fabrication method and heat treatment impact the thin film and nanocomposite structures and their technical applications, such as water purification, photodegradation, and solar cells [19,20,21,22,23,24,25,26,27,28]. The effect of annealing temperature on the optical characteristics of ITO thin films produced by direct current magnetron sputtering was investigated [29]. In contrast, the electrical properties of ITO thin films evaporated from two beryllia crucibles in the presence of oxygen were examined [30]. The influences of thermal annealing on the structural, linear/nonlinear optical, and electric sheet resistance of 350 nm thick ITO films made by the ball milling process and annealing temperature on the electrical and optical characteristics of a ~ 1.1 μm porous layer of ITO produced by spin coating were investigated [31, 32]. The structural and optical properties of ITO films with a thickness of 170 nm and varied in ratios deposited by the spray pyrolysis method were examined [33]. For commercial ITO with a thickness of 150 nm, the association of reflectance, skin depth, and plasmon frequency with conductivity was established [34]. The influence of annealing temperature on the structural, optical, and electrical properties of 200 nm thick ITO films produced by frequency magnetron sputtering was studied [35]. The optical properties of indium-doped and undoped 200 nm thick tin oxide films were investigated [36]. According to the previous literature, structural and thus electrical characteristics often depend on numerous factors such as annealing temperature, layer thickness, doping concentration, and fabrication process.
Consequently, thin layers of around 80 nm of the ITO film on a borofloat glass substrate are generated using the RF sputtering method. In addition, their electronic properties, such as linear and nonlinear optical parameters and electrical properties, are investigated at various annealing and measurement temperatures. The annealing process was performed at various temperatures, such as 473 K, 523 K, and 573 K. The study was conducted using multiple techniques such as X-ray diffraction, scanning electron microscope, four-probe measurements for electrical measurement, and the spectrophotometer to measure reflectance and transmittance in the wavelength range of 400–2400 nm.
2 Experimental details
RF sputtering was used to deposit ITO thin films with a thickness of 80 nm on 500 µm borofloat glass. The evaporation conditions were as follows: to achieve this thickness, the RF power was 100 W for 25 min of sputtering time (deposition rate: 3.2 nm/min). The base pressure of the chamber was 10–7 mbar, and the working pressure during sputtering was about 3 × 10–2 mbar. The borofloat glass substrates were cleaned by sonication with acetone and methanol, then were washed for 10 min with deionized water, and blown-dry with N2 gas. The films were annealed at 473 K, 523 K, and 573 K for 3 h in an oven under environmental conditions.
A Philips X-ray diffractometer of type-1710 with CuKα radiation of wavelength 1.5418 Å was used to examine the crystal structure of as-prepared and annealed ITO thin films. The scanning speed is 0.06°/min, and the diffraction angle (2θ) varies from 10° to 70° at room temperature (RT).
The surface morphologies of ITO thin films were investigated using a JEOL Type T200 scanning electron microscope (SEM). The SEM was set to a 15 kV acceleration voltage, and all samples were coated with a very thin gold coating before SEM examination. Image processing software (ImageJ) version 1.37 was used to examine the SEM images.
The DC electric conductivity (σ) of as-prepared ITO thin films was measured using a four-point probe arrangement throughout a temperature range of RT to 485 K. A Keithley 175A high internal impedance electrometer was used to examine the numerical values. Using the thermal evaporation approach under vacuum, thick Al electrodes with a square shape (5 mm × 5 mm) were deposited on the ITO films (105 Torr). More details about the electrical measurements may be obtained elsewhere [37].
A double-beam spectrophotometer, model SHIMADZU 2101, was used to measure the optical reflectance (R) and transmittance (T) of as-prepared and annealed ITO thin films. The spectrophotometer has a heat filter linked to a PC data collection system. The values of T and R were recorded in the wavelength range of 200–2500 nm.
3 Results and discussion
3.1 Structural and microstructure studies
Figure 1 shows the XRD diagrams of the electron beam evaporated ITO thin film annealed at various annealing temperatures. Only a single diffraction peak was observed for the as-prepared ITO and located at 2θ = 29.55° assigned to Miller indices of (222). The observed peak belongs to the cubic phase according to the JCPDS card number 01-089-4598, which agrees with the results in previous studies [38]. As the ITO samples were annealed at temperatures 473 and 523 K, the intensity of the diffraction peak at 2θ = 29.8° became greater, and an additional peak was observed at 2θ = 50.36°. The observed peak belongs to the cubic phase according to the JCPDS card number 01-089-4598. The annealing of ITO at 573 K leads to more crystallinity associated with an additional peak, in addition to the two later observed peaks, and this peak was observed at 2θ = 41.53°. The diffraction peaks were slightly moved to higher values when the annealing temperature was raised. The shift in the diffraction peaks to higher values is commonly attributed to the change in the lattice parameters and the average crystallite size. Using the well-known Scherrer equation [39], the average crystallite size of the cubic phase was estimated to be 19.08, 23.79, 21.69, and 18.64 nm for as-produced ITO, annealed at 473 K, 523 K, and 573 K, respectively.
Figure 2 shows the top-view SEM images for as-prepared and annealed ITO thin films. Various morphologies were detected based on the annealing temperature. The as-prepared sample contains nanoplates or nanosheets that are only partially attached. The annealing at 473 K increases the number of connected plates, and the entire sample comprises connected plates. An increase in annealing temperature to 523 K results in a new morphology constituting of dendritic morphology imprinted in the solid matrix. Annealing at 573 K produces spherical particles, some of which aggregate to create larger or related particles. The average estimated thickness of the produced plates is 80 nm and 130 nm for as-prepared and annealed samples at 473 K, respectively. Furthermore, the estimated size of the dendritic morphologies is 2 μm and 120 nm for black and white phases, and the estimated particle size for the spherical morphology is 0.5 μm.
3.2 Electrical parameters of ITO thin films
Figure 3a depicts the fluctuation of resistivity (ρ) as a function of measurement temperature (T) between room temperature and 485 K. It is well recognized that the resistivity of ITO films decreases fast at lower temperatures less than 370 K as the substrate temperature rises. Then, a slight decrease in resistivity was detected with an increase in the measuring temperature of more than 370 K. The value of ρ for the ITO thin films was reduced from 11.5 × 10–2 to 3.52 × 10–2 Ω cm as the temperature increased from 338 to 487 K, respectively. It was determined that the estimated values in the research are significantly lower than those obtained by other authors [31, 40]. The significant difference in this study might be attributed to the thinner ITO. The observed behaviors indicate that the substrate temperature influenced the electrical characteristics of the ITO films and resulted in a significant rise in the carrier's mobility and its concentration, revealing the ITO's semiconducting activity. Figure 3a depicts the sheet resistance \(({R}_{\text{s}})\) as a function of measurement temperature. The equation \({R}_{\text{s}}=\frac{\rho }{d}\) was used to compute the sheet resistance, where \(d\) is the sample thickness [41]. The indicated relationship behaves similarly to the electrical resistance. The value of \({R}_{\text{s}}\) of ITO thin films was reduced from 1.44 × 104 Ω to 4.4 × 103 Ω as the temperature had risen from 338 to 487 K, respectively.
The temperature dependence of the electrical conductivity (σ) for the as-prepared ITO thin films of 80 nm thickness deposited onto glass substrates is shown in Fig. 3b. The electrical conductivity is enhanced with the increase of the measuring temperature. As shown in the figure, three distinct regions could be noticed from the relation between ln(σ) and 103/T. In the first region (I) in the temperature range (335–350 K), the electrical conductivity value significantly increased with raising the temperature. The change in the electric conductivity with the sample temperature can be distinguished by the following Arrhenius relation [42]: \(\sigma \left(T\right)={\sigma }_{0}\text{exp}\left(\frac{-\Delta E}{{k}_{\text{B}}T}\right)\), where σ0 is the pre-exponential factor, kB is the Boltzmann constant, and T is the absolute temperature, and \(\Delta E\) is the activation energy for conduction. The second region (II) in the temperature range of 350–385 K represents that the electrical conductivity of the evaluated ITO films is slightly enhanced due to a slight rise in temperature, which corresponds to the transformation of the crystal structure. The third region (III) is in the temperature range of 385–500 K and shows a slight enhancement in the conductivity as the temperature rises. The estimated values of the electrical conductivity are equal to 16.3 meV, 1.1 meV, and 20.2 meV for regions I, II, and III, respectively.
3.3 Optical parameters of ITO thin films
In optoelectronics, the optical parameters of the used semiconductor are the vital parameters in the industry of those devices [43,44,45,46,47]. For instance, the transmittance (T), the reflectance (R), the absorption coefficient (α), the refractive index (n), and various parameters determine the validity of using this material. In the following section, those parameters will be briefly discussed.
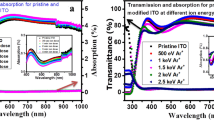
The values of T, R, and absorbance (A) of the as-prepared and annealed ITO thin film with 80 nm thickness at varying temperatures versus the incident wavelength ranging from 200 to 2500 nm are shown in Fig. 4a–c, respectively. From the observation, the transmittance of as-prepared ITO film was decreased as the annealing temperature was performed at 473 K and then increased when the temperature rises. The enhanced transmittance is convenient for optical device applications. This relates to an increase in the crystallinity of the film, which tends to reduce light scattering and defects, as previously stated [48, 49]. The ITO film annealed at 573 K reveals a maximum transmittance that reaches 95.7% at a wavelength of 1351.3 nm, as shown in Fig. 4a. Furthermore, the reflectance and absorbance of ITO film show an opposite trend, with reflectance and absorbance decreasing as temperature rises, which is also beneficial to the film's crystallinity. The absorbance of the film is calculated from the following equation: \(A=\sqrt{\text{ln}(\frac{1-R}{T})}\). Similarly, thermal annealing procedures elsewhere improve the optical transmittance of ITO films with different thicknesses (350 nm) [31].
The absorption coefficients (α), (αhν)2, and ln(α) versus the photon energy (hν) for ITO thin films at varying annealing temperatures are shown in Fig. 5a–c, respectively. The measurement of α is estimated from the following equation: \(\alpha = \frac{1}{d}\left[ {\ln \left( {\frac{{(1 - R)^{2} }}{{2T}} + \sqrt {\left( {\frac{{(1 - R)^{4} }}{{4T^{2} }} - R^{2} } \right)} } \right)} \right]\) [48], where d is the ITO film thickness. Values of α decrease with increasing temperature, as shown in Fig. 5a. From the observation, the absorption coefficient of heat-treated ITO films reveals a dependence on the photon energy (hν). Thus, the further rise in temperature will lead to a shift in the edge of the peak to a higher wavelength (red-shift).
The measurement of the optical bandgap is calculated from Tauc’s relationship (αhν) = αo (hν − Eopt.)m [49], where αo is Tauc parameter (dependent on the transition types), α is the absorption coefficient, m is the index (referring to optical transition type), and hν is the photon energy of incident light. Considering the direct allowable transition type between the bands of m = \(\frac{1}{2}\), the extrapolation of the linear part of the dependence of (αhν)2 on the hν is used to determine Eg from the intercept of extrapolated line to (αhν)2 = 0. As the temperature increases, Eg increases, which can be ascribed to the amplification of the crystallinity and reducing the defects concentration, leading to a reduction in the width of the localized states [50,51,52], as shown in Fig. 5b and Table 1. The value of the width of localized states \({E}_{\text{e}}\) is determined from the slope of the fitted lines associated with the Urbach equation (\(\alpha ={\alpha }_{\text{o}}{\text{e}}^{\frac{h\upnu }{{E}_{\text{e}}}}\)) [52]. From Fig. 5c, the \({E}_{\text{e}}\) value is 0.068, 1.18, 0.064, and 049 eV for ITO as-prepared at 473 K, 523 K, and 573 K, respectively. From Table 1, \({E}_{\text{e}}\) decreases with rising temperature, which could be related to the diminished in the disorders in the film.
The refractive index (n), the extension coefficient (kex), the optical surface resistance Rs, and the thermal emissivity \({\varepsilon }_{\text{th}}\) of ITO film are shown in Fig. 6a–d, respectively. The extension coefficient kex is calculated from the following equation: kex= \(\frac{\alpha \lambda }{4\pi }\) [53], where \(\lambda\) is the wavelength and α is the absorption coefficient. It was noticed that kex reduced with a rise in the annealing temperature; this decrease in kex is associated with the reduction in defect concentration, disorder [54], and carrier concentration [55]. The value of kex reveals a dependence on the wavelength and has 3 behaviors: (1) it decreased in the visible light region, (2) completely vanished in 703–1884 nm, and (3) took an upward and rapid increase, as shown in Fig. 5b. Values of n are determined from the following equation: \(n=\sqrt{\frac{4R}{{(R-1)}^{2}}-{k}_{ex}^{2}}+\frac{R+1}{R-1}\), where R is the reflectance and kex is the extension coefficient. It was noticed that n reduces with increasing temperature; this decrease refers to the decrease in the reflectance and the lattice shrinking. It is well known that transmittance and reflectance depend on the distribution of electrons or phonons. As the crystallinity improves and dislocations, impurities, and disorders decrease, increasing the annealing temperature causes photons to transmit with low scattering.
The optical surface resistance Rs and the thermal emissivity \({\varepsilon }_{\text{th}}\) of the as-prepared and heat-treated ITO film versus the wavelength are shown in Fig. 6c, d. The optical surface resistance and the thermal emissivity \({\varepsilon }_{\text{th}}\) are calculated from the following equations: \({R}_{\text{s}}=-\frac{4\pi }{c}\times \frac{1}{n\times \text{ln}(T)}\), \({\varepsilon }_{\text{th}}=1-\frac{1}{{\left[1+\left(2{\varepsilon }_{0}c\times {R}_{\text{s}}\right)\right]}^{2}}\), respectively, where c, n, and T refer to the speed of light, the refractive index, and the transmittance, respectively. It was noticed that Rs and \({\varepsilon }_{\text{th}}\) increased with increasing the wavelength before 1360 nm, exceeding that wavelength will show a decrease in Rs and\({\varepsilon }_{\text{th}}\). Furthermore, increasing the annealing temperature will lead to an increase in Rs and\({\varepsilon }_{\text{th}}\).
Optical density OD as a function of λ of the as-prepared and heat-treated ITO film versus λ is shown in Fig. 7. Values of the OD are calculated from \(\text{OD}=-{\text{log}}_{10}\left(T\right)=0.434\alpha d\) which represents the dependence of OD on the film thickness d (cm), the transmittance (T), and the absorption coefficient \(\alpha\). It was noticed that the optical density decreased with the temperature rise due to the rise in the transmittance and the improvement in the crystallinity with the temperature.
The dielectric constant components are significant for the calculation of various optical parameters, the imaginary\({\varepsilon }_{\text{i}}\), real part \({\varepsilon }_{\text{r}}\) of the dielectric constant, and the loss factor Tan(δ) of the as-prepared and heat-treated ITO film versus the incident wavelength λ, as shown in Fig. 8a–c, respectively. \({\varepsilon }_{\text{i}}\), \({\varepsilon }_{\text{r}}\), and Tan(δ) are calculated from \({\varepsilon }_{i}=2n{k}_{ex}\), \({\varepsilon }_{r}={n}^{2}-{k}_{ex}^{2}\), and Tan(δ) = \(\frac{{\varepsilon }_{\text{i }}}{{\varepsilon }_{\text{r}}}\), respectively, where n and kex refer to the refractive index and the extension coefficient, respectively.
The complex optical conductivity (σ ∗ = σ1 + iσ2) of the as-prepared and heat-treated ITO film versus the wavelength is shown in Fig. 9a, b. The real component σ1 and the imaginary component σ2 are calculated from the following equation: \({\sigma }_{1}=4\pi {\varepsilon }_{\text{o}}\frac{cn{k}_{ex}}{\lambda }\), and \({\sigma }_{2}=\frac{2\pi c{\varepsilon }_{\text{o}}}{\lambda }({n}^{2}-{k}_{ex}^{2})\), respectively, where c, λ, n, and kex refer to the speed of light, the wavelength, the refractive index, and the extension coefficient, respectively.
The volume energy loss function (VELF) and the surface energy loss function (SELF) of the as-prepared and heat-treated ITO film versus hν are shown in Fig. 10a, b. VELF indicates the energy loss according to the movement of the fast electron through the bulk material, SELF indicates the energy loss due to the movement of the fast electron through the surface of the material, and they together indicate the total energy loss. VELF and SELF are calculated from the following equations: VELF = \(\frac{{\varepsilon }_{i}^{2}}{{\varepsilon }_{i}^{2}+{\varepsilon }_{r}^{2}}\) and SELF = \(\frac{{\varepsilon }_{i}^{2}}{{\varepsilon }_{i}^{2}+{({\varepsilon }_{r}+1)}^{2}}\), where \({\varepsilon }_{\text{i}}\) is the imaginary and \({\varepsilon }_{\text{r}}\) is the real part of the dielectric constant. From the observation, VELF and SELF reveal a decreasing trend with rising temperatures.
The electrical susceptibility is χc of the as-prepared and heat-treated ITO film versus hν is shown in Fig. 11. χc is calculated from the following equation: \({\upchi }_{c}=\frac{1}{4\pi }({\varepsilon }_{r}-{n}^{2})\). It was noticed that χc increased with rising temperature, which reveals the trend of ITO film to be polarized in response to an external electric field, decreasing the total internal electric field inside it.
The plot of n2 of the as-prepared and heat-treated ITO film versus λ2 is shown in Fig. 12a. The values of the high-frequency dielectric constant ε∞ and \(\frac{N}{{m}^{*}}\) are determined from the Y-intercept and the slope of the fitted curves, which are estimated from the following equation: n2 = ε∞—(\(\frac{{e}^{2}}{4{\pi }^{2}{c}^{2}{\varepsilon }_{\text{o}}})(\frac{N}{{m}^{*}})\) λ2 [56], where e, εo, N, m*, and c refer to the electronic charge, the electrical permittivity for free space, the concentration of free carrier, the effective electron mass, and the speed of light, respectively. ε∞ and \(\frac{N}{{m}^{*}}\) values are listed in Table 1. From the observation, ε∞ and \(\frac{N}{{m}^{*}}\) reveal a decrease in their values with increasing the annealing temperature.
A plot of (n2 − 1)−1 of the as-prepared and heat-treated ITO film versus (hυ)2 is shown in Fig. 12b. The value of the average energy of the oscillator Eo and the average strength of interband optical transitions Ed are computed from the slope and intercept of the fitted curves estimated from the Wemple–DiDomenico (W-DD) relationship as follows: \({({n}^{2}-1)}^{-1}=\frac{{E}_{\text{o}}}{{E}_{\text{d}}}-\frac{{(h\upsilon )}^{2}}{{E}_{\text{o}}{E}_{\text{d}}}\) [57], which are listed in Table 1. From the observation, the values of Eo and Ed reveal an enhancement as the temperature rises.
4 Conclusion
The effects of annealing temperature on the crystal structure, linear/nonlinear optical characteristics, and the electrical properties of RF sputtered ITO film were investigated. The as-prepared and annealed ITO films had a cubic crystal structure with no evidence of contaminants. The thermally treated ITO thin film band has been found to influence crystallite size, hence changing their electrical and optical characteristics. The thermal annealing method was used to improve the transparency of ITO films. The optical energy bandgap increases as the crystallinity of the ITO films improves with increasing annealing temperature to 473 K. The optical constants and parameters, such as refractive index, optical density, extinction coefficient, SELF, and VELF, largely depend on the annealing temperature. The electrical resistance of the ITO film was found to decrease as the film temperature increased. The main findings show that thermal annealing of ITO film improves crystallite size, crystallinity, transparency in the visible region, and electrical conductivity, making annealed ITO film an excellent choice for optoelectronic devices and solar cells, among other potential applications.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
P.L. Praveen, J. Mol. Liq. 341, 117424 (2021). https://doi.org/10.1016/j.molliq.2021.117424
S. Chaudhuri, D. Bhattacharyya, A.B. Maity, A.K. Pal, Surface coatings for solar application, in Materials Science Forum, vol. 246 (Trans Tech Publications Ltd, Wollerau, 1997) pp. 181–206
C. Coutal, A. Azema, J.-C. Roustan, Thin Solid Films 288, 248–253 (1996). https://doi.org/10.1016/S0040-6090(96)08824-4
E. Benamar, M. Rami, C. Messaoudi, D. Sayah, A. Ennaoui, Sol. Energy Mater. Sol. Cells. 56, 125–139 (1999). https://doi.org/10.1016/S0927-0248(98)00151-2
S.H. Babu, S. Kaleemulla, N.M. Rao, C. Krishnamoorthi, J. Magn. Magn. Mater. 416, 66–74 (2016). https://doi.org/10.1016/j.jmmm.2016.05.007
H.R. Fallah, M. Ghasemi, A. Hassanzadeh, H. Steki, Mater. Res. Bull. 42(3), 487–496 (2007). https://doi.org/10.1016/j.materresbull.2006.06.024
C. Guillén, J. Herrero, J. Appl. Phys. 101, 073514 (2007). https://doi.org/10.1063/1.2715539
J. Kim, S. Shrestha, M. Souri, J.G. Connell, S. Park, A. Seo, Sci Rep. 10, 12486 (2020). https://doi.org/10.1038/s41598-020-69463-4
L. He, S.C. Tjong, Mater. Sci. Eng. R 109, 1–101 (2016). https://doi.org/10.1016/j.mser.2016.08.002
K.P. Kalyanikutty, G. Gundiah, C. Edem, A. Govindaraj, C.N.R. Rao, Chem. Phys. Lett. 408, 389–394 (2005). https://doi.org/10.1016/j.cplett.2005.04.037
M.J. Alamand, D.C. Cameron, Thin Solid Films 420, 76–82 (2002). https://doi.org/10.1016/S0040-6090(02)00737-X
A. Khan, F. Rahman, R. Nongjai, K. Asokan, Solid State Sci. 109, 106436 (2020). https://doi.org/10.1016/j.solidstatesciences.2020.106436
F.I. Ezema, C.D. Lokhande, R. Jose, Chemically Deposited Nanocrystalline Metal Oxide Thin Films: Synthesis, Characterizations, and Applications (Springer, Berlin, 2021). https://doi.org/10.1007/978-3-030-68462-4
M. Boehme, C. Charton, Surf. Coat. Technol. 200, 932–935 (2005). https://doi.org/10.1016/j.surfcoat.2005.02.040
H. Cho, Y.-H. Yun, Ceram. Int. 37, 615–619 (2011). https://doi.org/10.1016/j.ceramint.2010.09.033
Y.S. Jung, Thin Solid Films 467, 36–42 (2004). https://doi.org/10.1016/j.tsf.2004.02.047
L.-J. Meng, E. Liang, J. Gao, V. Teixeira, M.P.D. Santos, J. Nanosci. Nanotechnol. 9, 4151–4155 (2009). https://doi.org/10.1166/jnn.2009.M24
M. Woodhouse, D. Feldman, V. Ramasamy, B. Smith, T. Silverman, T. Barnes, J. Zuboy, R. Margolis, No. NREL/TP-7A40-80505. National Renewable Energy Lab. (NREL), Golden, CO (United States), 2021. https://www.nrel.gov/docs/fy22osti/80505.pdf
M.A. Mahdi, S.R. Yousefi, L.S. Jasim, M. Salavati-Niasari, Int. J. Hydrog. Energy. 47, 14319–14330 (2022). https://doi.org/10.1016/j.ijhydene.2022.02.175
S.R. Yousefi, H.A. Alshamsi, O. Amiri, M. Salavati-Niasari, J. Mol. Liq. 337, 116405 (2021). https://doi.org/10.1016/j.molliq.2021.116405
S.R. Yousefi, D. Ghanbari, M. Salavati-Niasari, M. Hassanpour, J. Mater. Sci. 27, 1244–1253 (2016). https://doi.org/10.1007/s10854-015-3882-6
S.R. Yousefi, M. Ghanbari, O. Amiri, Z. Marzhoseyni, P. Mehdizadeh, M. Hajizadeh-Oghaz, M. Salavati-Niasari, J. Am. Ceram. Soc. 104, 2952–2965 (2021). https://doi.org/10.1111/jace.17696
S.R. Yousefi, A. Sobhani, H.A. Alshamsi, M. Salavati-Niasari, RSC Adv. 11, 11500–11512 (2021). https://doi.org/10.1039/D0RA10288A
S.R. Yousefi, O. Amiri, M. Salavati-Niasari, Ultrason. Sonochem. 58, 104619 (2019). https://doi.org/10.1016/j.ultsonch.2019.104619
S.R. Yousefi, M. Masjedi-Arani, M.S. Morassaei, M. Salavati-Niasari, H. Moayedi, Int. J. Hydrog. Energy. 44, 24005–24016 (2019). https://doi.org/10.1016/j.ijhydene.2019.07.113
S.R. Yousefi, A. Sobhani, M. Salavati-Niasari, Adv. Powder Technol. 28, 1258–1262 (2017). https://doi.org/10.1016/j.apt.2017.02.013
S.R. Yousefi, D. Ghanbari, M. Salavati-Niasari, J. Nanostruct. 6, 80–85 (2016). https://doi.org/10.7508/jns.2016.01.00
P.L. Praveen, D.P. Ojha, Phase Transit. 87, 515–525 (2014). https://doi.org/10.1080/01411594.2013.852195
C.H.L. Weijtens, P.A.C. Van Loon, Thin Solid Films 196, 1–10 (1991). https://doi.org/10.1016/0040-6090(91)90169-X
M. Mizuhashi, Thin Solid Films 70, 91–100 (1980). https://doi.org/10.1016/0040-6090(80)90415-0
M. Ahmed, A. Bakry, A. Qasem, H. Dalir, Opt. Mater. 113, 110866 (2021). https://doi.org/10.1016/j.optmat.2021.110866
J. Ederth, P. Heszler, A. Hultåker, G.A. Niklasson, C.G. Granqvist, Thin Solid Films 445, 199–206 (2003). https://doi.org/10.1016/S0040-6090(03)01164-7
P.K. Manoj, B. Joseph, V.K. Vaidyan, D.S.D. Amma, Ceram. Int. 33, 273–278 (2007). https://doi.org/10.1016/j.ceramint.2005.09.016
S.H. Brewer, S. Franzen, J. Alloys Compd. 338, 73–79 (2002). https://doi.org/10.1016/S0925-8388(02)00217-7
N.M. Ahmed, F.A. Sabah, H.I. Abdulgafour, A. Alsadig, A. Sulieman, M. Alkhoaryef, Results Phys. 13, 102159 (2019). https://doi.org/10.1016/j.rinp.2019.102159
F.R. Chowdhury, S. Choudhury, F. Hasan, T. Begum, J. Bangladesh Acad. Sci. 35, 99–111 (2011). https://doi.org/10.3329/jbas.v35i1.7975
A.M. Abd-Elnaiem, M.A. Abdel-Rahim, S. Moustafa, J. Non-Cryst, Solids. 540, 120062 (2020). https://doi.org/10.1016/j.jnoncrysol.2020.120062
D.K. Kaushik, K.U. Kumar, A. Subrahmanyam, AIP Adv. 7, 015109 (2017). https://doi.org/10.1063/1.4974157
P. Scherrer, Nachr. Ges. Wiss. Göttingen. 26, 98–100 (1918). https://doi.org/10.1007/978-3-662-33915-2_7
F.H. Alsultany, N.M. Ahmed, M.Z. Matjafri, J. Nanosci. Lett. 4, 83 (2014). https://doi.org/10.4236/snl.2014.44012
M. Naftaly, S. Das, J. Gallop, K. Pan, F. Alkhalil, D. Kariyapperuma, S. Constant, C. Ramsdale, L. Hao, Electronics 10, 960 (2021). https://doi.org/10.3390/electronics10080960
A.K. Hassan, A.K. Ray, R.D. Gould, Phys. Status Solidi A 158, K23–K25 (1996). https://doi.org/10.1016/j.apsusc.2005.12.151
E. Muchuweni, T.S. Sathiaraj, H. Nyakotyo, Heliyon. 3, e00285 (2017). https://doi.org/10.1016/j.heliyon.2017.e00285
S. Rasool, K. Saritha, K.T.R. Reddy, M.S. Tivanov, A.V. Trofimova, S.E. Tikoto, L. Bychto, A. Patryn, M. Maliński, V.F. Gremenok, Curr. Appl. Phys. 19, 108–113 (2019). https://doi.org/10.1016/j.cap.2018.11.016
P.L. Praveen, J. Mol. Liq. 343, 117620 (2021). https://doi.org/10.1016/j.molliq.2021.117620
T.J. Jose, A. Simi, M.D. Raju, P.L. Praveen, Mol. Cryst. Liq. Cryst. 650, 46–55 (2017). https://doi.org/10.1080/15421406.2017.1328216
P.L. Praveen, D.P. Ojha, Phase Transit. 87, 64 (2014). https://doi.org/10.1080/01411594.2013.879478
M. El-Hagary, M. Emam-Ismail, E.R. Shaaban, A. Al-Rashidi, S. Althoyaib, Mater. Chem. Phys. 132, 581–590 (2012). https://doi.org/10.1016/j.matchemphys.2011.11.072
J. Tauc (ed.), Amorphous and Liquid Semiconductors (Springer, New York, 2012)
R. Panda, R. Naik, N.C. Mishra, J. Alloys Compd. 778, 819–826 (2019). https://doi.org/10.1016/j.jallcom.2018.11.142
D. Sahoo, P. Priyadarshini, A. Aparimita, D. Alagarasan, R. Ganesan, S. Varadharajaperumal, R. Naik, RSC Adv. 10, 26675–26685 (2020). https://doi.org/10.1039/D0RA04763E
I.S. Yahia, G.F. Salem, J. Iqbal, F. Yakuphanoglu, Phys. B Condens. Matter. 511, 54–60 (2017). https://doi.org/10.1016/j.physb.2017.01.030
D.J. Gravesteijn, Appl. Opt. 27, 736–738 (1988). https://doi.org/10.1364/AO.27.000736
R. Naik, A. Aparimita, D. Alagarasan, S. Varadharajaperumal, R. Ganesan, Opt. Quantum Electron. 52, 1–18 (2020). https://doi.org/10.1007/s11082-020-2245-6
H.M. Hosni, S.A. Fayek, S.M. El-Sayed, M. Roushdy, M.A. Soliman, Vacuum 81, 54–58 (2006). https://doi.org/10.1016/j.vacuum.2006.02.014
J.N. Zemel, J.D. Jensen, R.B. Schoolar, Phys. Rev. 140, A330 (1965). https://doi.org/10.1103/PhysRev.140.A330
S.H. Wemple, Phys. Rev. B. 7, 3767 (1973). https://doi.org/10.1103/PhysRevB.7.3767
Acknowledgements
The authors would like to express their gratitude to Prof. E. F. Abo Zeid (Assiut University, Egypt) for his assistance.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no external funding.
Author information
Authors and Affiliations
Contributions
AMAE contributed to investigation, writing—original draft, methodology, and formal analysis. AH contributed to writing—review and editing, administration, formal analysis, and investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This paper meets the ethical standards of this journal.
Consent to participate
All authors agree with the review of this paper in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd-Elnaiem, A.M., Hakamy, A. Influence of annealing temperature on structural, electrical, and optical properties of 80 nm thick indium-doped tin oxide on borofloat glass. J Mater Sci: Mater Electron 33, 23293–23305 (2022). https://doi.org/10.1007/s10854-022-09051-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09051-6