Abstract
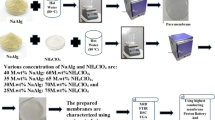
The development of high ion conducting membrane is in need of present time for efficient battery. The aim of this study is to develop a proton conducting polymer electrolyte using biopolymer sodium alginate (SA) with ammonium thiocyanate (NH4SCN) salt using solution casting technique. Addition of graphene quantum dot (GQD) with the highest conducting polymer electrolyte resulted with the increase in ionic conductivity of the polymer electrolyte. The prepared composition of SA: NH4SCN analyzed through XRD, FTIR, DSC, Ac impedance technique, LSV. SEM and TGA studies have been undertaken. The amorphous/crystalline natures of the obtained electrolytes were studied using the XRD technique. FTIR confirm the complex formation of salt and polymer. The glass transition temperature Tg has been obtained with the help of DSC. The highest proton conductivity of 8.72 × 10–3 S cm−1 has been obtained for the composition of 30 M.wt%SA: 70 M.wt%NH4SCN. The ionic conductivity has been improved to 2.22 × 10–2 S cm−1 duo to the addition of 0.75 ml GQD with 30 M.wt%SA: 70 M.wt%NH4SCN biopolymer electrolyte. Transference number analysis has been done using the Wagner’s polarization technique. Electrochemical stability value of 2.17 V (without GQD) and 2.83 V (with GQD) has been obtained using LSV. Proton conducting battery has been constructed using highest conducting biopolymer electrolyte and the open circuit voltage of 1.45 V (without GQD) and 1.48 V (with GQD) has been obtained. Construction of a single fuel cell has been made using highest proton conducting membrane providing an open circuit voltage 431 mV (without GQD) and 514 mV (with GQD).

























Similar content being viewed by others
Data availability
The datasets generated during the current study are not publicly available [still the paper is not to be published]. But the data available from the corresponding author on reasonable request.
References
Y.H. Liu, L.Q. Zhu, Y. Shi, Q. Wan, Proton conducting sodium alginate electrolyte laterally coupled low- voltage oxide—based transistors. Appl. Phys. Lett 104(133504), 1–4 (2014). https://doi.org/10.1063/1.4870078
H. Gao, K. Lian, Proton-conducting polymer electrolytes and their applications in solid supercapacitors a review. J. RSC Adv. 4, 33091–33113 (2014). https://doi.org/10.1039/C4RA05151C
A.F. Fuzlin, M.A. Saadiah, Y. Yao, Y. Nagao, A.S. Samsudin, Enhancing proton conductivity of sodium alginate doped with glycolic acid in bio-based polymer electrolytes system. J. Polym. Res. 27(207), 1–16 (2020). https://doi.org/10.1007/s10965-020-02142-0
A.F. Fuzlin, Y. Nagao, I.I. Misnon, A.S. Samsudin, Studies on structural and ionic transport in biopolymer electrolytes based alginate. Libr. Ionics 26, 1923–1938 (2020). https://doi.org/10.1007/s11581-019-03386-7
S. Selvalakshmi, T. Mathavan, S. Selvasekarapandian, M. Premalatha, Effect of ethylene carbonate plasticizer on agar-agar: NH4Br- based solid polymer electrolytes. J. Ionics 24, 2209–2217 (2018). https://doi.org/10.1007/s11581-017-2417-y
S.K. Vishaka, D.B. Kishor, S.R. Sudha, Natural polymers—a comprehensive review. J. Pharm. Biomed. Sci. 3(4), 1597–1613 (2012)
R. Manjuladevi, P.C. Selvin, S. Selvasekarapandian, R. Shilpa, V. Moniha, Lithium ion conducting biopolymer electrolyte based on pectin doped with lithium nitrate. AIP 1942(140075), 1–4 (2018). https://doi.org/10.1063/1.5029206
M. Premalatha, T. Mathavan, S. Selvasekarapandian, S. Monisha, S. Selvalakshmi, D.V. Pandi, Tamarind seed polysaccharide based li-ion conducting membranes. J. Ionics 23, 2677–2684 (2017). https://doi.org/10.1007/s11581-018-2541-3
V. Moniha, M. Alagar, S. Selvasekarapandian, B. Sundaresan, G. Boopathi, Conductive bio-polymer electrolyte i-carrageenan with ammonium nitrate for application in electrochemical devices. J. Non-Crystalline Solids 481, 424–434 (2018). https://doi.org/10.1016/j.jnoncrysol.2017.11.027
G.N. Devi, S. Chithra, S. Selvasekarapandian, M. Premalatha, S. Monisha, J. Saranya, Synthesis and characterization of dextrin based polymer electrolytes for potential applications in energy storage devices. J. Ionics 23, 3377–3388 (2017). https://doi.org/10.1007/s11581-017-2135-5
N. Shaari, S.K. Kamarudin, S. Basri, L.K. Shyuan, M.S. Masdar, D. Nordin, Enhanced mechanical flexibility and performance of sodium alginate polymer electrolyte bio-membrane for application in direct methanol fuel cell proton conductivity. J. Appl. Polym. Sci. 135, 46666 (2018). https://doi.org/10.1002/app.46666
O.S. Reddy, M.C.S. Subha, T. Jithendra, C. Madhavi, K.C. Rao, Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. (2020). https://doi.org/10.1016/j.jpha.2020.07.002
P.R.S. Reddy, K. Madhusudana, K.S.V.K. Rao, Y. Shchipunov, H. Chang-Sik, Synthesis of alginate based silver nanocomposite hydrogels for biomedical applications. J. Macromol. Res. 22, 832–842 (2014). https://doi.org/10.1007/s13233-014-2117-7
B.K. Satheeshababu, I. Mohamed, Synthesis and characterization of sodium alginate conjugate and study of effect of conjugation on drug release from matrix tablet. Indian J. Pharm. Sci. 77(5), 579–585 (2015)
S.T. Prajapati, L.D. Patel, D.M. Patel, studies on formulation and in vitro evaluation of floating matrix tablets of domperidone. Indian J. Pharm. Sci. 71, 19–23 (2009). https://doi.org/10.4103/0250-474X.51944
A. Salisu, M.M. Sanagi, A. Abu Naim, W.A.W. Ibrahim, K.J.A. Karim, Removal of lead ions from aqueous solutions using sodium alginate-graft-poly (methyl methacrylate) beads. J. Desalin. Water Treat. 57(33), 15353–15361 (2015). https://doi.org/10.1080/19443994.2015.1071685
Y.O. Iwaki, M. Hernandezescalona, J.R. Briones, A. Pawlicka, Sodium alginate based ionic conducting membranes. Mol. Cryst. Liq. Cryst. 554, 221–231 (2012). https://doi.org/10.1080/15421406.2012.634329
S. Mohanapriya, S.D. Bhat, A.K. Sahu, A. Manokaran, R. Vijayakumar, S. Pitchumani, P. Sridhar, A.K. Shukla, Sodium alginate based proton exchange membranes as electrolyte for DMFCs. Energy Environ. Sci. 3, 1746–1756 (2010). https://doi.org/10.1039/C0EE00033G
N. Shaari, S.K. Kamarudin, Characterization studies of sodium alginate/sulfonated grapheme oxide based polymer electrolyte membrane for direct methanol fuel cell. Malays. J. Anal. Sci. 21, 113–118 (2017)
F. Hajifathaliha, A. Mahboubi, L. Nematollahi, E. Mohit, N. Bolourchian, Comparison of different cationic polymers efficacy in fabrication of alginate multilayer microcapsules. Asian J. Pharm. Sci. 15, 95–103 (2018). https://doi.org/10.1016/j.ajps.2018.11.007
N.M.J. Rasali, Y. Nagao, A.S. Samsudin, Enhacement on amorphous phase in solid biopolymer electrolyte based alginate doped NH4NO3. Ionics 25, 641–654 (2018). https://doi.org/10.1007/s11581-018-2667-3
L. Aguero, D. Zaldivar-Silva, L. Pena, M.L. Dias, Alginate microparticles as oral colon drug delivery device. A review. Carbohydr. Polym. 168, 32–43 (2017). https://doi.org/10.1016/j.carbpol.2017.03.033
J.G.O. Filho, J.M. Rodrigues, A.C.F. Valadares, A.B. Almeida, T.M. Lima, K.P. Takeuchi, Active food packaging: alginate films with cottonseed protein hydrolysates. Food Hydrocolloids 92, 267–275 (2019). https://doi.org/10.1016/j.foodhyd.2019.01.052
N. Isıklan, G. Küçükbalcı, Microwave-induced synthesis of alginate–graft-poly(N-isopropylacrylamide) and drug release properties of dual pH- and temperature-responsive beads. Eur. J. Pharm. Biopharm. 82(2), 316–331 (2012). https://doi.org/10.1016/j.ejpb.2012.07.015
J.H. Li, Y. Huang, Role of alginate in antibacterial finishing of textiles. Int. J Biol. Macromol. 94, 466–473 (2017). https://doi.org/10.1016/j.ijbiomac.2016.10.054
S.B. Bae, H.C. Nam, W.H. Park, Electrospraying of environmentally sustainable alginate microbeads for cosmetic additives. Int. J. Biol. Macromol. 133, 278–283 (2019). https://doi.org/10.1016/j.ijbiomac.2019.04.058
S. Park, R. Ruoff, Nature chemical methods for the production of graphenes. Nanotechnol 4(4), 217–224 (2009). https://doi.org/10.1038/nnano.2009.58
N.R. Ko, M. Nafiujjaman, J.S. Lee, H.N. Lim, Y.K. Lee, I.K. Kwon, Graphene quantum dot-based theranostic agents for active targeting of breast cancer. RSC Adv. 7, 11420–11427 (2017). https://doi.org/10.1039/C6RA25949A
J. Shen, Y. Zhu, X. Yang, C. Li, Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 48, 3686–3699 (2012). https://doi.org/10.1039/C2CC00110A
X. Wang, X. Sun, J. Lao, H. He, T. Cheng, M. Wang, S. Wang, F. Huang, Multifunctional graphene quantum dots for simultaneous targetedcellular imaging and drug delivery. Colloids Surf. B 122, 638–644 (2014). https://doi.org/10.1016/j.colsurfb.2014.07.043
T. Maheshwari, K. Tamilarasan, S. Selvasekarapandian, R. Chitra, M. Muthukrishnan, Synthesis and characterization of Dextran, poly (vinyl alcohol) blend biopolymer electrolytes with NH4NO3, for electrochemical applications. Int. J. Green Energy 19, 314–330 (2021). https://doi.org/10.1080/15435075.2021.1946811
N.F. Mazuki, A.F. Fuzlin, M.A. Saadiah, A.S. Samsudin, An investigation on the abnormal trend of the conductivity properties of CMC/PVA-doped NH4Cl-based solid biopolymer electrolyte system. J. Ionics 25(6), 2657–2667 (2019). https://doi.org/10.1007/s11581-018-2734-9
M.I.H.A. Sohaimy, M.I.N.M. Isa, Natural inspired carboxymethyl cellulose (CMC) doped with ammonium carbonate (AC) as biopolymer electrolyte. J. Polym. 12(11), 2487 (2020). https://doi.org/10.3390/polym12112487
R. Hemalatha, M. Alagar, S. Selvasekarapandian, B. Sundaresan, V. Moniha, G. Boopathi, P.C. Selvin, Preparation and characterization of proton-conducting polymer electrolyte based on PVA, amino acid proline, and NH4Cl and its applications to electrochemical devices. J. Ionics 25(10), 141–154 (2019). https://doi.org/10.1007/s11581-018-2564-9
G. Boopathi, S. Pugalendhi, S. Selvasekarapandian, M. Premalatha, S. Monisha, G. Aristatil, Development of proton conducting biopolymer membrane based on agar–agar for fuel cell. J. Ionics 23, 2781–2790 (2017). https://doi.org/10.1007/s11581-016-1876-x
S. Monisha, T. Mathavan, S. Selvesekarapandian, A.M.F. Beniala, G. Aristatil, N. Manic, M. Premalatha, D.V. Pandi, Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr. Polym. 157, 38–47 (2017). https://doi.org/10.1016/j.carbpol.2016.09.026
A.F. Fuzlin, A.S. Samsudin, Studies on favorable ionic conduction and structural properties of biopolymer electrolytes system-based alginate. J. Polym. Bull. 78, 2155–2175 (2021). https://doi.org/10.1007/s00289-020-03207-2
P. Karthika, V. Sasikala, B. Sundaresan, Analysis of conductance spectra and traneference number measurements on polyvinyl chloride—ammonium thiocyanate polymer electrolyte added with SrTiO3. Shanlax Int. J. Arts Sci. Hum. 5, 347–351 (2017)
C. Chen, D. Zhao, T. Hu, J. Sun, X. Yang, Highly fluorescent nitrogen and sulfur co-doped graphene quantum dots for an inner filter effect-based cyanide sensor. Sens. Actuators B 241, 779–788 (2017). https://doi.org/10.1016/j.snb.2016.11.010
R.M. Hodge, G.H. Edward, G.P. Simon, Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37, 1371 (1996). https://doi.org/10.1016/0032-3861(96)81134-7
W. Chen, Q. Feng, G. Zhang, Q. Yang, C. Zhang, The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 113, 1–7 (2017). https://doi.org/10.1016/j.mineng.2017.07.016
M. Aprilliza, Characterization and properties of Sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conf. Ser. Mater. Sci. Eng. 188, 12019 (2017). https://doi.org/10.1088/1757-899X/188/1/012019
P. Kanti, K. Srigowri, J. Madhuri, B. Smitha, S. Sridhar, Dehydration of ethanol through blend membranes of chitosan and sodium alginate by pervaporation. Sep. Puri. Technol. 40, 259–266 (2004). https://doi.org/10.1016/j.seppur.2004.03.003
A.F. Fuzlin, N.A. Bakri, B. Sahraoui, A.S. Samsudin, Study on the effect of lithium nitrate in ionic conduction properties based alginate biopolymer electrolytes. Mater. Res. Express 7(1), 015902 (2020). https://doi.org/10.1088/2053-1591/ab57bb
M.I. Diana, P.C. Selvin, S. Selvasekarapandian, M.V. Krishna, Investigations on Na-ion conducting electrolyte based on sodium alginate biopolymer for all-solid-state sodium-ion batteries. J. Solid State Electrochem. 25:2009–2020 (2021). https://doi.org/10.1007/s10008-021-04985-z
M. Premalatha, T. Mathavan, S. Selvasekarapandian, S. Monisha, D.V. Pandi, S. Selvalakshmi, Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J. Non-Cryst. Solids 453, 131–140 (2016). https://doi.org/10.1016/j.jnoncrysol.2016.10.008
V. Moniha, M. Alagar, S. Selvasekarapandian, B. Sundaresan, R. Hemalatha, G. Boopathi, Synthesis and characterization of bio-polymer electrolyte base in iota-carrageenan with ammonium Thiocyanate and its application. J. Solid State Electrochem 22(10), 1–15 (2018)
C. N. Unnisa, S. Chithra, S. Selvasekarapandian, S. Monisha, G. N. Devi, V. Moniha, M. Hema, Development of poly(glycerol suberate) polyester (PGS)-PVA blend polymer electrolytes with NH4SCN and its application. Ionics 24:1979–1993 (2018). https://doi.org/10.1007/s11581-018-2466-x
N. Zhang, J. Xu, X. Gao, X. Fu, D. Zheng, Factors affecting water resistance of alginate/gellan blend films on paper cups for hot drinks. Carbohydr. Polym. 156, 435–442 (2017). https://doi.org/10.1016/j.carbpol.2016.08.101
P. Treenate, P. Monvisade, In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 99, 71–78 (2017). https://doi.org/10.1016/j.ijbiomac.2017.02.061
Z. Tong, Y. Chen, Y. Liu, L. Tong, J. Chu, K. Xiao, Z. Zhou, W. Dong, X. Chu, Preparation, characterization and properties of alginate/poly (γ-glutamic acid) composite microparticles. Mar. Drugs 15(4), 91 (2017). https://doi.org/10.3390/md15040091
P. Perumal, P.C. Selvin, S. Selvasekarapandian, Characterization of biopolymer pectin with lithium chloride and its applications to electrochemical devices. J. Ionics (2018). https://doi.org/10.1007/s11581-018-2507-5
B.A. Boukamp, A package of impedance admittance data analysis. Solid State Ionics 18&19, 136–140 (1986). https://doi.org/10.1016/0167-2738(86)90100-1
S. Karthikeyan, S. Sikkanthar, S. Selvasekarapandian, D. Arunkumar, H. Nithya, Y. Iwa, J. Kawamura, Structural, electrical and electrochemical properties of polyacrylonitrile-ammonium hexaflurophosphate polymer electrolyte system. J. Polym. Res. 23, 51 (2016). https://doi.org/10.1007/s10965-016-0952-2
S. Selvalakshmi, T. Mathavan, S. Selvasekarapandian, M. Premalatha, A study of electrochemical devices based on agar-agar NH4I biopolymer electrolytes. AIP (2017). https://doi.org/10.1063/1.5029150
J.B. Wagner, C.J. Wagner, Electrical conductivity measurements on curprous halidas. J. Chem. Phys. 26, 1597–1601 (1957). https://doi.org/10.1063/1.1743590
S. Karthikeyan, S. Selvasekarapandian, M. Premalatha, S. Monisha, G. Boopathi, G. Aristatil, A. Arun, S. Madeswaran, Proton conducting I-Carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 10, 2775–2780 (2016). https://doi.org/10.1007/s11581-016-1901-0
T. Maheshwari, K. Tamilarasan, S. Selvasekarapandian, R. Chitra, S. Kiruthika, Investigation of blend biopolymer electrolytes based on Dextran-PVA with ammonium thiocyanate. J. Solid State Electrochem. 25(3), 755–765 (2021). https://doi.org/10.1007/s10008-020-04850-5
K. Pandey, N. Lakshmi, S. Chandra, A rechargeable solid state proton battery with an intercalating cathode and an anode containing a hydrogen storage-material. J. Power Sources 76(1), 116–123 (1998). https://doi.org/10.1016/S0378-7753(98)00132-3
P. Christopher, P. Perumal, S. Selvasekarapandian, A. Monisha, G. Boopathi, M.V.L. Chandra, Study of proton-conducting polymer electrolyte based on K-carrageenan and NH4SCN for electrochemical devices. Ionics 24, 1–8 (2018). https://doi.org/10.1007/s11581-018-2521-7
R.M. Naachiyar, M. Ragam, S. Selvasekarapandian, M.V. Krishna, P. Buvaneshwari, Development of biopolymer electrolyte membrane using Gellan gum biopolymer incorporated with NH4SCN for electrochemical application. Ionics 27, 3415–3429 (2021). https://doi.org/10.1007/s11581-021-04095-w
M. Aprilliza, Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conf. Ser. 188(1), 012019 (2017). https://doi.org/10.1088/1757-899X/188/1/012019
Funding
The authors declare that no funds grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Entire work done by [NV] and full manuscript written by [NV]. The full Manuscript corrected by [CS]. The concept of the work given by [SS]. Fuel cell work has been done by [MVK]. N-S based Graphene quantum dot synthesized by [KN].
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vanitha, N., Shanmugapriya, C., Selvasekarapandian, S. et al. Investigation of N–S-based graphene quantum dot on sodium alginate with ammonium thiocyanate (NH4SCN) biopolymer electrolyte for the application of electrochemical devices. J Mater Sci: Mater Electron 33, 14847–14867 (2022). https://doi.org/10.1007/s10854-022-08404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08404-5