Abstract
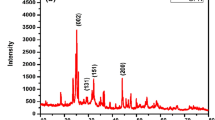
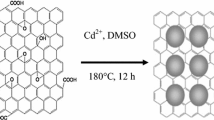
Reduced graphene oxide/cadmium meta niobate (RGOCN) hybrid nanocomposite was hydrothermally synthesised and subjected to structural characterization through X-ray diffraction and various microscopic analysis. Pure CN exhibited a cubical, rectangular slab like mixed morphology with average particle size ranging from 50 nm to 2 μm. Further, this work revealed that RGOCN composite showed exfoliation of graphene sheets with mixed morphology of cubes and rectangular slab. Spectral analysis enabled in understanding the various functional groups present in RGOCN. The optical band gap of prepared RGOCN composite material was estimated as 4.024 eV. The calculated remanent polarization and coercive electric field of cadmium niobate (CN) and RGOCN are Pr = 0.0151 µC cm−2, Ec = − 1.30 kV cm−1 and Pr = 0.0685 µC cm−2, Ec = − 2.39 kV cm−1, respectively. RGOCN was developed into an electrode material and its electrochemical behaviour was evaluated by adopting the cyclic voltammetry and galvanostatic charging discharging measurements. The observed specific surface area of hybrid RGOCN showed enhanced redox current rate than the pseudo-capacitive CN. Also, hybrid RGOCN electrode furnished enhancement in specific capacitance for different scan rates and current density than CN. Specific capacitance of RGOCN was estimated to be 58.2 F g−1 for very low molar H2SO4 electrolyte at a current density of 1 A g−1. Furthermore, RGOCN coated over carbon electrode furnished cyclic stability with 98% of capacitive retention after 500 cycles at 2 A g−1. The maximum energy density of 8.08 Wh kg−1 with consuming high power density of 4137 W kg−1 at 1 A g−1 compared with few other reported materials make graphene nested CN nanocomposite stay as a significant material supporting energy storage applications.











Similar content being viewed by others
References
F.Z. Amir, V.H. Pham, E.M. Schultheis, J.H. Dickerson, Flexible, all-solid-state, high-cell potential supercapacitors based on holey reduced graphene oxide/manganese dioxide nanosheets. Electrochim. Acta 260, 944–951 (2018)
C. Ogata, R. Kurogi, K. Awaya, K. Hatakeyama, T. Taniguchi, M. Koinuma, Y. Matsumoto, All-graphene oxide flexible solid-state supercapacitors with enhanced electrochemical performance. ACS Appl. Mater. Interfaces 9(31), 26151–26160 (2017)
A.K. Geim, K.S. Novoselov, The rise of graphene. Nat. Mater. 6, 183–191 (2007)
A. Garcia, A.F.B. Lopera, N. Ornelas-Soto, A.M. Garay-Tapia, F.R. Pérez, Á. Salazar, A general strategy for direct synthesis of reduced graphene oxide by chemical exfoliation of graphite. Mater. Chem. Phys. 218, 51–61 (2018)
M. Batzill, The surface science of graphene: metal interfaces, CVD synthesis, nanoribbons, chemical modifications and defects. Surf. Sci. Rep. 67, 83–115 (2012)
D.R. Dreyer, The chemistry of graphene oxide, in Graphene Oxide: Reduction Recipes, Spectroscopy and Applications (Springer, Cham, 2015), pp. 61–95
I.S. El-Hallag, M.N. El-Nahass, S.M. Youssry, R. Kumar, M.M. Abdel-Galeil, A. Matsuda, Facile in-situ simultaneous electrochemical reduction and deposition of reduced graphene oxide embedded palladium nanoparticles as high performance electrode materials for supercapacitor with excellent rate capability. Electrochim. Acta 314, 124–134 (2019)
R. Kumar, S. Sahoo, E. Joanni, R.K. Singh, K. Maegawa, W.K. Tan, G. Kawamura, K.K. Kar, A. Matsuda, Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 39, 47–65 (2020)
R. Kumar, S. Sahoo, E. Joanni, R.K. Singh, W.K. Tan, K.K. Kar, A. Matsuda, Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 75, 100786 (2019)
R. Kumar, S. Sahoo, E. Joanni, R.K. Singh, R.M. Yadav, R.K. Verma, D.P. Singh, W.K. Tan, A. Pérez, S.A. del Pino, A. Moshkalev, Matsuda, A review on synthesis of graphene, h-BN and MoS2 for energy storage applications: Recent progress and perspectives. Nano Res. 12, 2655–2694 (2019)
F. Li, X. Jiang, J. Zhao, S. Zhang, Graphene oxide: a promising nanomaterial for energy and environmental applications. Nanomater. Energy 16, 488–515 (2015)
W. Deng, X. Ji, M. Gomez-Mingot, F. Lu, Q. Chen, C.E. Banks, Chem. Commun. 48, 2770 (2012)
E. Mitchell, J. Candler, F. De Souza, R.K. Gupta, B.K. Gupta, L.F. Dong, High performance supercapacitor based on multilayer of polyaniline and graphene oxide. Synth. Met. 199, 214–218 (2015)
R. Imran Jafri, N. Rajalakshmi, S. Ramaprabhu, Nitrogen doped graphene nano platelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Mater. Chem. 20, 7114–7117 ( (2010), , )
V. Dhand, K.Y. Rhee, H.J. Kim, D.H. Jung, A comprehensive review of graphene nanocomposites: research status and trends J. Nanomater., Article ID 763953 (2013)
R. Kumar, S. Youssry, K.Z. Ya, W.K. Tan, G. Kawamura, A. Matsuda, Microwave-assisted synthesis of Mn3O4-Fe2O3/Fe3O4@rGO ternary hybrids and electrochemical performance for supercapacitor electrode. Diam. Relat. Mater. 101, 107622 (2019)
R. Kumar, R.K. Singh, A.R. Vaz, R. Savu, S.A. Moshkalev, Self-assembled and one-step synthesis of interconnected 3D network of Fe3O4/reduced graphene oxide nanosheets hybrid for high-performance supercapacitor electrode. ACS Appl. Mater. Interfaces 9(10), 8880–8890 (2017)
R. Kumar, R.K. Singh, R. Savu, P.K. Dubey, P. Kumar, S.A. Moshkalev, Microwave- assisted synthesis of void-induced graphene-wrapped nickel oxide hybrids for supercapacitor applications. RSC Adv. 6, 26612–26620 (2016)
X. Chen, H. Fan, Y. Fu, L. Liu, J. Chen, Low-temperature fabrication and crystallization behavior of Pb(Mg1/3Nb2/3O3) crystallites by a hydrothermal process. J. Alloys Compd. 469(1-2), 322–326 (2009)
M. Rahimi-Nasrabadi, V. Pourmohamadian, M.S. Karimi, H.R. Naderi, M.A. Karimi, K. Didehban, M.R. Ganjali, Assessment of supercapacitive performance of europium tungstate nanoparticles prepared via hydrothermal method. J Mater. Sci. Mater Electron. 28, 12391–12398 (2017)
H.R. Naderi, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, M.R. Ganjali, Decoration of nitrogen-doped reduced graphene oxide with cobalt tungstate nanoparticles for use in high-performance supercapacitors. Appl. Surf. Sci. 423, 1025–1034 (2017)
A. Sobhani-Nasab, M. Rahimi-Nasrabadi, H.R. Naderi, V. Pourmohamadian, F. Ahmadi, M.R. Ganjali, H. Ehrlich, Sonochemical synthesis of terbium tungstate for developing high power supercapacitors with enhanced energy densities. Ultrason. Sonochem. 45, 189–196 (2018)
J. Li, M. Ostling, Prevention of graphene restacking for performance boost of supercapacitors—a review. Crystals 3, 163 (2013)
U. Patil, S.C. Lee, S. Kulkarni, J.S. Sohn, M.S. Nam, S. Han, S.C. Jun, Nanostructured pseudocapacitive materials decorated 3D graphene foam electrodes for next generation supercapacitors. Nanoscale 7, 6999 (2015)
C. An, K. Tang, C. Wang, G. Shen, Y. Jin, Y. Qian, Characterization of LiNbO3 nanocrystals prepared via a convenient hydrothermal route. Mater. Res. Bull. 37, 1791–1796 (2002)
A.Z. Simões, A.H.M. González, A. Ries, M.A. Zaghete, B.D. tojanovic, J.A. Varela, Influence of thickness on crystallization and properties of LiNbO3 thin films. Mater. Charact. 50, 239–244 (2003)
M. Liu, D. Xue, An efficient approach for the direct synthesis of lithium niobate powders. Solid State Ionics 177, 275–280 (2006)
J. Liu, I. Shakir, D.J. Kang, Lithium niobate nanoflakes as electrodes for highly stable electrochemical supercapacitor devices. Mater. Lett. 119, 84–87 (2014)
C. Daniela, D.V. Marcano, J.M. Kosynkin, A. Berlin, Z. Sinitskii, S.A. Slesarev, L.B. Alemany, W. Lu, J.M. Tour, Improved synthesis of graphene oxide. ACS Nano 4(8), 4806–4814 (2010)
M.I.S. Kumar, S. Shahil Kirupavathy, E. Jerusha, S. Sureshkumar, M. Vinolia, Synthesis and characterization of novel reduced graphene oxide supported barium niobate (RGOBN) nanocomposite with enhanced ferroelectric properties and thermal stability. J. Mater. Sci. Mater. Electron. 29, 19228–19237 (2018)
X. Jiao, Y. Qiu, L. Zhang, X. Zhang, Comparison of the characteristic properties of reduced graphene oxides synthesized from natural graphites with different graphitization degrees. RSC Adv. 7(82), 52337–52344 (2017)
R.F. Samigullina, T.I. Krasnenko, M.V. Rotermel, A.P. Tyutyunnik, S.G. Titova, O.M. Fedorova, Crystal-chemical and physicochemical properties of complex cadmium oxides with pyrochlore and columbite type of structure. Mater. Chem. Phys. 168, 122–126 (2015)
S.H. Huh, in Thermal Reduction of Graphene Oxide, Physics and Applications of Graphene—Experiments, ed. by S. Mikhailov (InTechOpen, London, 2011). ISBN: 978-953-307-217-3
K. Venkateswarlu, M. Sandhyarani, T.A. Nellaippan, N. Rameshbabu, Estimation of crystallite size, lattice strain and dislocation density of nanocrystalline carbonate substituted hydroxyapatite by X-ray peak variance analysis. Procedia Mater. Sci. 5, 212–221 (2014)
B. Fultz, J.M. Howe 2002, High-resolution TEM imaging, in Transmission Electron Microscopy and Diffractometry of Materials (Springer, Berlin). https://doi.org/10.1007/978-3-662-04901-3_10
X. Gou, B. Wang, H. Liu, J. Yao, G. Wang, J. Yang, J. Park, Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 112, 8192–8195 (2008)
Y.-W. Hsu, T.-K. Hsu, C.-L. Sun, Y.-T. Nien, N.-W. Pu, M.-D. Ger, Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim. Acta 82, 152–157 (2012)
D. Das, A.K. Ganguli, Design of nanostructured cadmium tantalate and niobate and their photocatalytic properties. RSC Adv. 3, 21697–21705 (2013)
J. Sengupta, K. Das, U.N. Nandi, C. Jacob, Substrate free synthesis of graphene nanoflakes by atmospheric pressure chemical vapour deposition using Ni powder as a catalyst. Bull. Mater. Sci. 42(4), 136 (2019)
S. Ratha, C.S. Rout, Supercapacitor electrodes based on layered tungsten disulfide-reduced graphene oxide hybrids synthesized by a facile hydrothermal method. ACS Appl. Mater. Interfaces 5, 11427–11433 (2013)
T. Jiao, Y. Liu, Y. Wu, Q. Zhang, X. Yan, F. Gao, A.J.P. Bauer, J. Liu, T. Zeng, B. Li, Facile and scalable preparation of graphene oxide-based magnetic hybrids for fast and highly efficient removal of organic dyes. Sci. Rep. 5, 12451 (2015)
K. Krishnamoorthy, R. Mohan, S.-J. Kim, Graphene oxide as a photocatalytic material. Appl. Phys. Lett. 98, 244101–244103 (2011)
L.A. Bugaev, V.A. Shuvaeva, I.B. Alekseenko, K.N. Zhuchkov, R.V. Vedrinskii, Determination of the local structure of NbO6 octahedra in the orthorhombic phase of a KNbO3 crystal using EXAFS. Phys. Solid State 40(6), 1001–1005 (1998)
C. Balamurugan, S. Arunkumar, D.-W. Lee, Hierarchical 3D nanostructure of GdInO3 and reducedgraphene-decorated GdInO3 nanocomposite for CO sensing applications. Sensors Actuators Chem. 234, 155–166 (2016)
R.F. Samigullina, M.V. Rotermel, I.V. Nikolaenko, T.I. Krasnenko, Phase Equilibria in the Nb2O5–CdO System and the Thermal Stability of Cd2Nb2O7 and CdNb2O6. Russ. J. Inorg. Chem. 61(2), 156–160 (2016)
F. Soofivand, M. Salavati-Niasari, Co3O4/Graphene nanocomposite: pre-graphenization synthesis and photocatalytic investigation of various magnetic nanostructures. RSC Adv. 5, 64346–64353 (2015)
J. Wu, X. Shen, L. Jiang, K. Wang, K. Chen, Solvothermal synthesis and characterization of sandwich-like graphene/ZnO Nanocomposites. Appl. Surf. Sci. 256, 2826–2830 (2010)
C.A. Díaz-Moreno, J.A. López, Y. Ding, A.H. Macias, C. Li, R. B. Wicker, Multiferroic and optical properties of La0.05Li0.85NbO3 and LiNbO3 nanocrystals. J. Nanotechnol. 2018(2), 1–13 (2018)
S. Acharya, J. Mondal, S. Ghosh, S.K. Roy, P.K. Chakrabarti, Multiferroic behaviour of Lanthanum ortho ferrite (LaFeO3). Mater. Lett. 64, 415–418 (2010)
L. Vaisman, H.D. Wagner, G. Marom, The role of surfactants in dispersion of carbon nanotubes. Adv. Coll. Interface. Sci. 128-130, 37–46 (2006)
M. Zhang, Y. Wang, H. Liu, T. Ma, J. Xie, S. Shao, Controllable synthesis of CoNb2O6 nanoparticles on multifunctional sulfur and phosphorus dual-doped graphene as advanced electrodes for hybrid supercapacitors. Electrochim. Acta 309, 104–115 (2019)
Z. Li, J. Wang, S. Liu, X. Liu, S. Yang, Synthesis of hydrothermallyreduced Graphene/MnO2 composites and their electrochemical properties as supercapacitors. J. Power Sources 196, 8160–8165 (2011)
K. Hareesh, B. Shateesh, J.F. Williams, K. Asokan, D.M. Phase, K. Priya Madhuri, S.K. Haram, S.D. Dhole, Enhanced supercapacitance behaviour of low energy ion beam reduced graphene oxide. Mater. Res. Express 4, 065018 (2017)
D.M.G.T. Nathan, S.J.M. Boby, P. Basu, R. Mahesh, S. Harish, S. Joseph, P. Sahayaraj, One-pot hydrothermal preparation of Cu2O-CuO/rGOnanocomposites with enhanced electrochemical performance forsupercapacitor applications. Appl. Surf. Sci. 449, 474–484 (2018)
K. Siwatch, K. Sharma, A. Arora, S.K. Tripathi, Review of supercapacitors: materials and devices. J. Energy Storage 21, 801–825 (2019)
R. Kumar, H.-J. Kim, S. Park, A. Srivastava, I.-K. Oh, Graphene-wrapped and cobalt oxide-intercalated hybrid for extremely durable super-capacitor with ultrahigh energy and power densities. Carbon 79, 192–202 (2014)
R. Kumar, R. Matsuo, K. Kishida, M.M. Abdel-Galeil, Y. Suda, A. Matsuda, Homogeneous reduced graphene oxide supported NiO-MnO2 ternary hybrids for electrode material with improved capacitive performance. Electrochim. Acta 303, 246–256 (2019)
R. Kumar, R.K. Singh, A.R. Vaz, R. Savu, S.A. Moshkalev, Self-assembled and one-step synthesis of interconnected 3D network of Fe3O4/reduced graphene oxide nanosheets hybrid for high-performance supercapacitor electrode. ACS Appl. Mater. Interfaces 9, 8880–8890 (2017)
S. Maiti, A.K. Das, S.K. Karan, B.B. Khatua, Carbon nanohorn-graphene nanoplate hybrid: an excellent electrode material for supercapacitor application. J. Appl. Polym. Sci. 132, 42118–42124 (2015)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, M.I.S., Kirupavathy, S.S. Investigations on the capacitive behaviour of hydrothermally synthesised cadmium meta niobate incorporated reduced graphene oxide hybrid nanocomposite electrode material. J Mater Sci: Mater Electron 33, 2428–2449 (2022). https://doi.org/10.1007/s10854-021-07450-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-07450-9