Abstract
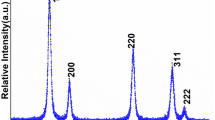
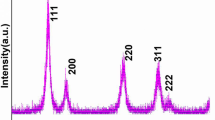
Nickel-substituted ceria nanoparticles (CeO2:Ni NPs) were prepared by the co-precipitation process under environmental conditions. X-ray diffraction (XRD), field emission-transmission electron microscopy, UV/visible, X-ray photoelectron spectroscopy, cyclic voltammetry, and electrochemical impedance spectroscopy techniques were successfully used to investigate the crystallographic structure, phase purity, morphology, optical properties, chemical composition, and electrocatalytic properties of the as-prepared ceria NPs. XRD pattern shows the formation of single-phase, highly crystalline, and cubic phase nanostructure with an average of 10 nm crystalline size. As observed from TEM micrographs, particles were highly aggregated may be due to the synthesis in aqueous media. The electrochemical properties and sensing performance of the CeO2:Ni NPs pasted on glassy carbon electrode were measured against different thiourea concentrations. The fabricated electrode revealed excellent electrocatalytic activity against thiourea concentrations as well as characterization in comparison to the bare electrode. The electrode exhibited a linear detection range between 3.56 and 1000 µM, detection limit 3.56 µM, and sensitivity 2.52 µA mL µM−1 cm−2 with regression coefficient 0.995. The electrochemical stability, chemical kinetic, and reproducibility were also examined in the presence of thiourea in phosphate buffer solution.








Similar content being viewed by others
References
X. Wang, Y.D. Li, Chem.—Eur. J 9, 5627 (2003). https://doi.org/10.1002/chem.200304785
J.Y. Luo, M. Meng, J.S. Yao et al., Appl. Catal. B—Environ. 87, 92 (2009). https://doi.org/10.1016/j.apcatb.2008.08.017
A.A. Ansari, J.P. Labis, M. Alam, S.M. Ramay, N. Ahmad, A. Mahmood, Acta Metall. Sin.—Engl. Lett. 29, 265 (2016). https://doi.org/10.1007/s40195-016-0387-0
H. Yen, Y. Seo, S. Kaliaguine, F. Kleitz, Angew. Chem. 124, 12198 (2012)
J. Zhang, J. Guo, W. Liu et al., Eur. J. Inorg. Chem. 2015, 969 (2015)
A.A. Ansari, A. Kaushik, P.R. Solanki, B.D. Malhotra, Electrochem. Commun. 10, 1246 (2008). https://doi.org/10.1016/j.elecom.2008.06.003
A.A. Ansari, P.R. Solanki, B.D. Malhotra, Appl. Phys. Lett. (2008). https://doi.org/10.1063/1.2953686
P.R. Solanki, C. Dhand, A. Kaushik, A.A. Ansari, K.N. Sood, B.D. Malhotra, Sens. Actuators B-Chem. 141, 551 (2009). https://doi.org/10.1016/j.snb.2009.05.034
A. Kaushik, P.R. Solanki, A.A. Ansari, S. Ahmad, B.D. Malhotra, Nanotechnology (2009). https://doi.org/10.1088/0957-4484/20/5/055105
S. Patil, S. Seal, Y. Guo, A. Schulte, J. Norwood, Appl. Phys. Lett. (2006). https://doi.org/10.1063/1.220795
C.K. Kim, T. Kim, I.Y. Choi et al., Angew. Chem. Int. Ed Engl. 51, 11039 (2012). https://doi.org/10.1002/anie.201203780
R. Khan, A. Kaushik, P.R. Solanki, A.A. Ansari, M.K. Pandey, B.D. Malhotra, Anal. Chim. Acta 616, 207 (2008). https://doi.org/10.1016/j.aca.2008.04.010
A. Ali, A.A. Ansari, A. Kaushik et al., Mater. Lett. 63, 2473 (2009). https://doi.org/10.1016/j.matlet.2009.08.038
A.A. Ansari, J. Labis, M. Alam, S.M. Ramay, N. Ahmad, A. Mahmood, J. Chin. Chem. Soc. 62, 925 (2015). https://doi.org/10.1002/jccs.201500195
A.A. Ansari, J. Labis, M. Alam, S.M. Ramay, N. Ahmad, A. Mahmood, J. Electroceram. 36, 150 (2016). https://doi.org/10.1007/s10832-016-0018-1
A.A. Ansari, J. Labis, M. Alam, S.M. Ramay, N. Ahmad, A. Mahmood, Phase Transit. 89, 261 (2016). https://doi.org/10.1080/01411594.2015.1116532
X. Zhang, J. Wei, H. Yang et al., Eur. J. Inorg. Chem. 2013, 4443 (2013). https://doi.org/10.1002/ejic.201300370
Q. Tan, C. Du, Y. Sun, L. Du, G. Yin, Y. Gao, J. Power Sources 263, 310 (2014). https://doi.org/10.1016/j.jpowsour.2014.04.062
C.S.S. Pavan Kumar, R. Pandeeswari, B.G. Jeyaprakash, J. Alloys Compd. 602, 180 (2014). https://doi.org/10.1016/j.jallcom.2014.02.143
A.A. Ansari, P.R. Solanki, B.D. Malhotra, J. Biotechnol. 142, 179 (2009). https://doi.org/10.1016/j.jbiotec.2009.04.005
S. Maensiri, C. Masingboon, P. Laokul et al., Cryst. Growth Des. 7, 950 (2007). https://doi.org/10.1021/cg0608864
M. Leoni, R. Di Maggio, S. Polizzi, P. Scardi, J. Am. Ceram. Soc. 87, 1133 (2004). https://doi.org/10.1111/j.1551-2916.2004.01133.x
Z. Wang, Z. Quan, J. Lin, Inorg. Chem. 46, 5237 (2007). https://doi.org/10.1021/ic0701256
J. Wang, Z. Li, S. Zhang et al., Sens. Actuators, B Chem. 255, 862 (2018). https://doi.org/10.1016/j.snb.2017.08.149
A.A. Ansari, M. Alam, M.A. Ali, Colloids Surf. A Physicochem. Eng. Asp. 613, 126116 (2021). https://doi.org/10.1016/j.colsurfa.2020.126116
Z.L. Wang, G.R. Li, Y.N. Ou, Z.P. Feng, D.L. Qu, Y.X. Tong, J. Phys. Chem. C 115, 351 (2011). https://doi.org/10.1021/jp1070924
A.A. Ansari, A. Kaushik, J. Semicond. 31, 033001 (2010). https://doi.org/10.1088/1674-4926/31/3/033001
A.A. Ansari, S.P. Singh, B.D. Malhotra, J. Alloy. Compd. 509, 262 (2011). https://doi.org/10.1016/j.jallcom.2010.07.009
D. Channei, B. Inceesungvorn, N. Wetchakun et al., Ceram. Int. 39, 3129 (2013). https://doi.org/10.1016/j.ceramint.2012.09.093
A.A. Ansari, J. Semicond. 31, 053001 (2010). https://doi.org/10.1088/1674-4926/31/5/053001
Y.-W. Zhang, R. Si, C.-S. Liao, C.-H. Yan, C.-X. Xiao, Y. Kou, J. Phys. Chem. B 107, 10159 (2003). https://doi.org/10.1021/jp034981o
A.A. Ansari, J.P. Labis, M. Alam, S.M. Ramay, N. Ahmed, A. Mahmood, Anal. Lett. 50, 1360 (2017). https://doi.org/10.1080/00032719.2016.1218499
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University, Riyadh, for funding this work through Research Group No. RG-1439-089.
Author information
Authors and Affiliations
Contributions
AAA: conceptualization, methodology, investigation, resources, data curation, writing—original draft, writing—review & editing, supervision, project administration, and funding acquisition. MA: methodology, formal analysis, and data curation.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any competing or financial interest in the present manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ansari, A.A., Alam, M. Nickle-ion-substituted ceria nanoparticles-based electrochemical sensor for sensitive detection of thiourea. J Mater Sci: Mater Electron 32, 23266–23274 (2021). https://doi.org/10.1007/s10854-021-06811-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06811-8