Abstract
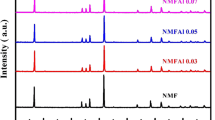
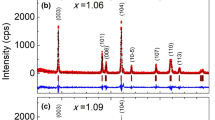
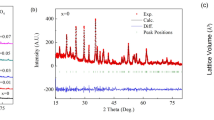
We successfully fabricated Na2/3Mn1/2Fe1/2−xAlxO2, where x = 0, 0.01, … 0.10, by a modified solid-state reaction technique. The structural properties of the Al-substituted samples were investigated by x-ray diffraction (XRD), scanning electron microscope (SEM), Fourier transform infrared spectroscopy and x-ray absorption fine structure (XAFS) measurements. It was found that there were no impurity phases in the XRD patterns of the samples and they fit the P63/mmc symmetry. The Al substitution in Na2/3Mn1/2Fe1/2O2 causes a decrease in the a-lattice parameter, but the c-parameter starts to increase after a certain substitution value of Al. We suggest that a certain proportion of Al in the samples triggers the change of the spin configuration of the Fe ions, and it may cause an increase in the lattice parameters. The size of the grains was found to be less than 0.9 µm, from SEM images for all samples. The valence states of the substituted samples as well as the local structure around Fe and Mn were investigated by means of XAFS measurements. The highest capacity for the first cycle was obtained as 134.3 mAh/g for x = 0.07, and the best capacity fade was found to be 0.23 for x = 0.08 substitution. So, the highest performance of the Al-substituted cells was found when 0.08 ≥ x ≥ 0.06. The environmental temperature effects on the battery cells were determined at 10 ºC, room temperature and 50 ºC, and it was found that the temperature plays a crucial role in the Na-ion batteries.









Similar content being viewed by others
References
N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada, S. Komaba, P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. (2012). https://doi.org/10.1038/nmat3309
J. Xu, S.L. Chou, J.L. Wang, H.K. Liu, S.X. Dou, Layered P2-Na0.66Fe0.5Mn0.5O2 cathode material for rechargeable sodium-ion batteries. ChemElectroChem (2014). https://doi.org/10.1002/celc.201300026
J.K. Park, G.G. Park, H.H. Kwak, S.T. Hong, J.W. Lee, Enhanced rate capability and cycle performance of titanium-substituted P2-type Na0.67Fe0.5Mn0.5O2 as a cathode for sodium-ion batteries. ACS Omega 3, 361–368 (2017). https://doi.org/10.1021/acsomega.7b01481
S. Chu, Y. Chen, J. Wang, J. Dai, K. Liao, W. Zhou, Z. Shao, A cobalt and nickel co-modified layered P2-Na2/3Mn1/2Fe1/2O2 with excellent cycle stability for high-energy density sodium-ion batteries. J. Alloys Compd. (2019). https://doi.org/10.1016/j.jallcom.2018.10.150
B. Mortemard de Boisse, D. Carlier, M. Guignard, C. Delmas, Structural and electrochemical characterizations of P2 and new O3-NaxMn1-yFeyO2 phases prepared by auto-combustion synthesis for Na-ion batteries. J. Electrochem. Soc. (2013). https://doi.org/10.1149/2.032304jes
G. Singh, J.M. López, M. Del Amo, S. Galceran, T. Pérez-Villar, Rojo, Structural evolution during sodium deintercalation/intercalation in Na2/3[Fe1/2Mn1/2]O2. J. Mater. Chem. A. (2015). https://doi.org/10.1039/c4ta06360k
V. Duffort, E. Talaie, R. Black, L.F. Nazar, Uptake of CO2 in Layered P2-Na0.67Mn0.5Fe0.5O2: insertion of carbonate anions. Chem. Mater (2015). https://doi.org/10.1021/acs.chemmater.5b00097
R. Malik, D. Burch, M. Bazant, G. Ceder, Particle size dependence of the ionic diffusivity. Nano Lett. (2010). https://doi.org/10.1021/nl1023595
M.H. Han, B. Acebedo, E. Gonzalo, P.S. Fontecoba, S. Clarke, D. Saurel, T. Rojo, Synthesis and electrochemistry study of P2- and O3-phase Na2/3Fe1/2Mn1/2O2. Electrochim. Acta. (2015). https://doi.org/10.1016/j.electacta.2015.10.003
M. Guan, J. Chen, X. Zhang, L. Yang, B. Wang, S. Zhong, Yarn-ball-shaped P2-Na2/3Fe1/2Mn1/2O2 nanofibers prepared by magnetic-assisted electrospinning method as high-performance cathode material for Na-ion batteries. Mater. Lett. (2019). https://doi.org/10.1016/j.matlet.2019.07.001
R. Viswanatha, B. Kishore, U. Bharath, N. Munichandraiah, Communication—electrochemical investigation of plate-like Na2/3Fe1/2Mn1/2O2 for sodium ion cathode. J. Electrochem. Soc. 165, A263–A265 (2018). https://doi.org/10.1149/2.0911802jes
Y. Sui, Y. Hao, X. Zhang, S. Zhong, J. Chen, J. Li, L. Wu, Spray-drying synthesis of P2-Na2/3Fe1/2Mn1/2O2 with improved electrochemical properties. Adv. Powder Technol. 31, 190–197 (2020). https://doi.org/10.1016/j.apt.2019.10.010
E. Altin, S. Altundag, S. Altin, A. Bayri, Fabrication of Cr doped Na0.67Fe0.5Mn0.5O2 compounds and investigation of their structural, electrical, magnetic and electrochemical properties. J. Mater. Sci. Mater. Electron. (2019). https://doi.org/10.1007/s10854-019-02136-9
J. Chen, S. Zhong, X. Zhang, J. Liu, S. Shi, Y. Hu, L. Wu, High performance of hexagonal plates P2- Na2/3Fe1/2Mn1/2O2 cathode material synthesized by an improved solid-state method. Mater. Lett. (2017). https://doi.org/10.1016/j.matlet.2017.05.084
Y. Bai, L. Zhao, C. Wu, H. Li, Y. Li, F. Wu, Enhanced sodium ion storage behavior of P2-Type Na2/3Fe1/2Mn1/2O2 synthesized via a chelating agent assisted route. ACS Appl. Mater. Interfaces. 8, 2857–2865 (2016). https://doi.org/10.1021/acsami.5b11848
C. Ding, T. Nohira, R. Hagiwara, Charge-discharge performance of Na2/3Fe1/2Mn1/2O2 positive electrode in an ionic liquid electrolyte at 90 °C for sodium secondary batteries. Electrochim. Acta (2017). https://doi.org/10.1016/j.electacta.2017.02.069
I. Moeez, H.G. Jung, H.D. Lim, K.Y. Chung, Presodiation strategies and their effect on electrode-electrolyte interphases for high-performance electrodes for sodium-ion batteries. ACS Appl. Mater. Interfaces. (2019). https://doi.org/10.1021/acsami.9b14381
H. Wang, R. Gao, Z. Li, L. Sun, Z. Hu, X. Liu, Different effects of Al substitution for Mn or Fe on the structure and electrochemical properties of Na0.67Mn0.5Fe0.5O2 as a sodium ion battery cathode material. Inorg. Chem. (2018). https://doi.org/10.1021/acs.inorgchem.8b00284
E. Marelli, C. Villevieille, S. Park, N. Hérault, C. Marino, Co-Free P2-Na0.67Mn0.6Fe0.25Al0.15O2 as promising cathode material for sodium-ion batteries. ACS Appl. Energy Mater. 1, 5960–5967 (2018). https://doi.org/10.1021/acsaem.8b01015
W. Kong, H. Wang, L. Sun, C. Su, X. Liu, Understanding the synergic roles of MgO coating on the cycling and rate performance of Na0.67Mn0.5Fe0.5O2 cathode. Appl. Surf. Sci. (2019). https://doi.org/10.1016/j.apsusc.2019.143814
B.H. Toby, EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. (2001). https://doi.org/10.1107/S0021889801002242
B.H. Toby, R.B. Von Dreele, The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013). https://doi.org/10.1107/S0021889813003531
K. Kubota, S. Komaba, Review—practical issues and future perspective for Na-Ion batteries. J. Electrochem. Soc. (2015). https://doi.org/10.1149/2.0151514jes
H. Su, S. Jaffer, H. Yu, Transition metal oxides for sodium-ion batteries. Energy Storage Mater. (2016). https://doi.org/10.1016/j.ensm.2016.06.005
R. Zhang, Y. Cui, W. Fan, G. He, X. Liu, Ambient stable Na0.76Mn0.48Ti0.44O2 as anode for Na-ion battery. Electrochim. Acta (2019). https://doi.org/10.1016/j.electacta.2018.10.126
M. Augustin, D. Fenske, I. Bardenhagen, A. Westphal, M. Knipper, T. Plaggenborg, J. Kolny-Olesiak, J. Parisi, Manganese oxide phases and morphologies: a study on calcination temperature and atmospheric dependence. Beilstein J. Nanotechnol. (2015). https://doi.org/10.3762/bjnano.6.6
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A. (1976). https://doi.org/10.1107/S0567739476001551
M.A.C. Nascimento, The nature of the chemical bond. J. Braz. Chem. Soc. (2008). https://doi.org/10.1590/S0103-50532008000200007
L.H. Ahrens, The use of ionization potentials Part 1. Ionic radii of the elements. Geochim. Cosmochim. Acta (1952). https://doi.org/10.1016/0016-7037(52)90004-5
F. Ahangaran, A. Hassanzadeh, S. Nouri, Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int. Nano Lett (2013). https://doi.org/10.1186/2228-5326-3-23
M. Dimitrakopoulou, X. Huang, J. Kröhnert, D. Teschner, S. Praetz, C. Schlesiger, W. Malzer, C. Janke, E. Schwab, F. Rosowski, H. Kaiser, S. Schunk, R. Schlögl, A. Trunschke, Insights into structure and dynamics of (Mn, Fe)O: X-promoted Rh nanoparticles. Faraday Discuss. (2018). https://doi.org/10.1039/c7fd00215g
J. Liang, D. Wei, Q. Cheng, Y. Zhu, X. Li, L. Fan, J. Zhang, Y. Qian, Cycling of Fe2O3 nanorice as an anode throughelectrochemical porousness and the solid–electrolyteinterphase thermolysis approach. ChemPlusChem 79, 143–150143 (2014)
L. Ren, H. Qiu, W. Qin, M. Zhang, Y. Li, P. Wei, Inhibition mechanism of Ca2+, Mg2+ and Fe3+ in finecassiterite flotation usingoctanohydroxamic acid. R. Soc. open sci 5, 180158 (2018)
A. Yuan, X. Wang, Y. Wang, J. Hu, Textural and capacitive characteristics of MnO2 nanocrystals derivedfrom a novel solid-reaction route. Electrochim. Acta 54, 1021–1026 (2009)
V.T. Le, T.M. Pham, V.D. Doan, O.E. Lebedeva, H.T. Nguyen, Removal of Pb (ii) ions from aqueous solution using a novel composite adsorbent of Fe3O4/PVA/spent coffee grounds. Sep. Sci. Technol. 54(18), 3070–3081 (2019)
V.G.P. Ribeiro, A.C.H. Barreto, J.C. Denardin, G. Mele, L. Carbone, S.E. Mazzetto, E.M.B. Sousa, P.B.A. Fechine, Magnetic nanoparticles coated with anacardic acid derived from cashew nut shell liquid. J. Mater. Sci. 48, 7875–7882 (2013)
C.M. Julien, M. Massot, Vibrational Spectroscopy Of Electrode Materials For Rechargeable Lithium Batteries Iii. Oxide Frameworks. In: Proceedings of the International Workshop “Advanced Techniques for Energy Sources Investigation and Testing” 4–9 Sept. (2004), Sofia, Bulgaria
C. Liu, K. Shih, Y. Gao, F. Li, L. Wei, Dechlorinating transformation of propachlor through nucleophilic substitution by dithionite on the surface of alumina. J. Soils Sediments (2012). https://doi.org/10.1007/s11368-012-0506-0
M.D. Abràmoff, P.J. Magalhães, S.J. Ram, Image processing with imageJ. Biophotonics Int (2004). https://doi.org/10.1201/9781420005615.ax4
T.J. Collins, ImageJ for microscopy, Biotechniques. (2007). https://doi.org/10.2144/000112517
C.A. Schneider, W.S. Rasband, K.W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. (2012). https://doi.org/10.1038/nmeth.2089
E. Talaie, V. Duffort, H.L. Smith, B. Fultz, L.F. Nazar, Structure of the high voltage phase of layered P2-Na2/3-z[Mn1/2Fe1/2]O2 and the positive effect of Ni substitution on its stability. Energy Environ. Sci. (2015). https://doi.org/10.1039/c5ee01365h
R. Zhan, L. Hu, H. Han, C. Dai, J. Jiang, M. Xu, Exploration of Mn0.5Ti2(PO4)3@rgo composite as anode electrode for Na-ion battery. J. Mater. Sci. Mater. Elect. 29, 4250–4255 (2018). https://doi.org/10.1007/s10854-017-8370-8
C. Wang, Y. Yang, Z. Chen, C. He, J. Su, Y. Wen, A mild process for the synthesis of Na2Ti3O7 as an anode material for sodium-ion batteries in deep eutectic solvent. J. Mater. Sci. Mater. Elect. 30, 8422–8427 (2019). https://doi.org/10.1007/s10854-019-01159-6
J.-H. Hong, M.-Y. Wang, Y.Y. Du, L. Deng, G. He, The role of Zn substitution in P2-type Na0.67Ni0.23Zn0.1Mn0.67O2 cathode for inhibiting the phase transition at high potential and dissolution of manganese at low potential. J. Mater. Sci. Mater. Elect. 30, 4006–4013 (2019). https://doi.org/10.1007/s10854-019-00687-5
Acknowledgements
Authors would like to thank TUBITAK for financial support by the contract number of 119M169. Prof. S. Altin would like to thank SESAME for XAFS experiments for granting beamtime to the proposal with project number of 20185036. We also want to thank EU CALIPSO programme for travelling and accommodation support during the SESAME experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altin, S., Altundağ, S., Altin, E. et al. An investigation of the improvement in energy storage performance of Na2/3Mn1/2Fe1/2O2 by systematic Al-substitution. J Mater Sci: Mater Electron 31, 14784–14794 (2020). https://doi.org/10.1007/s10854-020-04042-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04042-x