Abstract
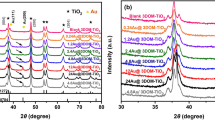
Recently, we have successfully achieved the atmospheric pressure catalytic synthesis of nanodiamonds by applying high stress to Ti3C2/Ni composite and then performing chlorine gas etching at atmospheric pressure. In this work, we chose three different catalysts (Fe, Co and Ni), with Ti3C2 as the precursor, aiming to investigate the effect of catalyst type on the synthesis of NDs. The results show that all three catalysts can catalyze the synthesis of NDs and that the crystal type and plasticity of the catalysts may have an important effect on the type and size of the resulting ND. When Fe and Ni were used as catalysts, the percentage of C-type NDs obtained was higher than that of C-type NDs catalyzed by Co (hexagonal close-packed crystal structure) due to its face-centered cubic crystal structure. In contrast, more H-type NDs were catalytically synthesized when Co (hexagonal close-packed crystal structure) was used as the catalyst. In addition, the number of small-size NDs (< 10 nm) catalytically synthesized by Co was significantly more than that catalytically synthesized by Fe and Ni, this may be caused by the poor plasticity of Co with a hexagonal close-packed crystal structure compared to Fe and Ni with a face-centered cubic crystal structure.









Similar content being viewed by others
Data and code availability
The data presented in this study are available on request from the corresponding author.
References
Kossovsky N, Gelman A, Hnatyszyn HJ, Rajguru S, Garrell RL, Torbati S, Freitas SSF, Chow GM (1995) Surface-modified diamond nanoparticles as antigen delivery vehicles. Bioconjugate Chem 6:507–511. https://doi.org/10.1021/bc00035a001
Chkhalov NI, Fedorchenko MV, Kruglyakov EP, Volokhov AI, Baraboshkin KS, Komarov VF, Kostyukov SI, Petrov EA (1995) Ultradispersed diamond powders of detonation nature for polishing X-ray mirrors. Nucl Instrum Methods Phys Res Sect A 359:155–156. https://doi.org/10.1016/0168-9002(94)01387-X
Irifune T, Kurio A, Sakamoto S, Inoue T, Sumiya H (2003) Ultrahard polycrystalline diamond from graphite. Nature 421:599–600. https://doi.org/10.1038/421599b
Mochalin VN, Shenderova O, Ho D, Gogotsi Y (2012) The properties and applications of nanodiamonds. Nat Nanotechnol 7:11–23. https://doi.org/10.1038/nnano.2011.209
Greiner NR, Phillips DS, Johnson JD, Volk F (1988) Diamonds in detonation soot. Nature 333:440–442. https://doi.org/10.1038/333440a0
Thiel MV, Ree FH (1987) Properties of carbon clusters in TNT detonation products: graphite-diamond transition. J Appl Phys 62:1761–1767. https://doi.org/10.1063/1.339575
Hirai H, Kondo K (1991) Modified phases of diamond formed under shock compression and rapid quenching. Science 253:772–774. https://doi.org/10.1126/science.253.5021.7
Vlasov II, Shenderova OA (2014) Detonation nanodiamonds: science and applications. Pan Stanford, Boca Raton. https://doi.org/10.1201/b15541
Boudou JP, Curmi PA, Jelezko F, Wrachtrup J, Aubert P, Sennour M, Balasubramanian G, Reuter R, Thorel A, Gaffet E (2009) High yield fabrication of fluorescent nanodiamonds. Nanotechnology 20:235602. https://doi.org/10.1088/0957-4484/20/23/235602
Banhart F, Ajayan PM (1996) Carbon onions as nanoscopic pressure cells for diamond formation. Nature 382:433–435. https://doi.org/10.1038/382433a0
Xiao J, Li JL, Liu P, Yang GW (2014) A new phase transformation path from nanodiamond to new-diamond via an intermediate carbon onion. Nanoscale 6:15098–15106. https://doi.org/10.1039/c4nr05246c
Kumar A, Lin PA, Xue A, Hao BY, Yap YK, Sankaran RM (2013) Formation of nanodiamonds at near-ambient conditions via microplasma dissociation of ethanol vapour. Nat Commun 4:2618. https://doi.org/10.1038/ncomms3618
Kamali AR, Fray DJ (2015) Preparation of nanodiamonds from carbon nanoparticles at atmospheric pressure. Chem Commun 51:5594–5597. https://doi.org/10.1039/c5cc00233h
Hemawan KW, Gou H, Hemley RJ (2015) Diamond synthesis at atmospheric pressure by microwave capillary plasma chemical vapor deposition. Appl Phys Lett 107:181901. https://doi.org/10.1063/1.4934751
Manjarrez A, Zhou K, Chen CQ, Tzeng YK, Cai LL (2022) Atmospheric-pressure flame vapor deposition of nanocrystalline diamonds: implications for scalable and cost-effective coatings. ACS Appl Nano Mater 5:10715–10723. https://doi.org/10.1021/acsanm.2c02059
Zhang W, Fan B, Zhang Y, Fan J (2017) Hydrothermal synthesis of well crystallized C8and diamond nanocrystals and pH-controlled C8↔ diamond phase transition. CrystEngComm 19:1248–1252. https://doi.org/10.1039/c6ce02635d
Chmiola J, Yushin G, Dash R, Gogotsi Y (2006) Effect of pore size and surface area of carbide derived carbons on specific capacitance. J Power Sources 158:765–772. https://doi.org/10.1016/j.jpowsour.2005.09.008
Presser V, Heon M, Gogotsi Y (2011) Carbide-derived carbons–from porous networks to nanotubes and graphene. Adv Funct Mater 21:810–833. https://doi.org/10.1002/adfm.201002094
Tallo I, Thomberg T, Kontturi K, Jänes A, Lust E (2011) Nanostructured carbide-derived carbon synthesized by chlorination of tungsten carbide. Carbon 49:4427–4433. https://doi.org/10.1016/j.carbon.2011.06.033
Dutzer MR, Mangarella MC, Schott JA, Dai S, Walton KS (2017) The effects of reactor design on the synthesis of titanium carbide-derived carbon. Chem Eng Sci 160:191–199. https://doi.org/10.1016/j.ces.2016.11.019
Tomas CD, Suarez-Martinez I, Vallejos-Burgos F, López MJ, Kaneko K, Marks NA (2017) Structural prediction of graphitization and porosity in carbide-derived carbons. Carbon 119:1–9. https://doi.org/10.1016/j.carbon.2017.04.004
Peng B, Zhang Y, Wang GH, Yuan L, Jia R (2018) Ferromagnetism observed in silicon-carbide-derived carbon. Phys Rev B 97:054401. https://doi.org/10.1103/PhysRevB.97.054401
Yushin GN, Hoffman EN, Nikitin A, Ye H, Barsoum MW, Gogotsi Y (2005) Synthesis of nanoporous carbide-derived carbon by chlorination of titanium silicon carbide. Carbon 43:2075–2082. https://doi.org/10.1016/j.carbon.2005.03.014
Urbonaite S, Juárez-Galán JM, Leis J, Rodríguez-Reinoso F, Svensson G (2008) Porosity development along the synthesis of carbons from metal carbides. Microporous Mesoporous Mater 113:14–21. https://doi.org/10.1016/j.micromeso.2007.10.046
González-García P, Urones-Garrote E, Ávila-Brande D, Gómez-Herrero A, Otero-Díaz LC (2010) Structural study of carbon nanomaterials prepared by chlorination of tungsten carbide and bis(cyclopentadienyl)tungsten dichloride. Carbon 48:3667–3675. https://doi.org/10.1016/j.carbon.2010.06.003
Sui J, Lu JJ (2010) Synthesis of carbon nitride powder by selective etching of TiC0.3N0.7 in chlorine-containing atmosphere at moderate temperature. Mater Chem Phys 123:264–268. https://doi.org/10.1016/j.matchemphys.2010.04.008
Zhang Z, Ma HX, Zhang JJ, Li S, Zhang RJ (2023) Nickel-catalyzed synthesis of nano-diamonds during the high-temperature chlorination of the layered structured bulk of Ti3C2 under atmospheric pressure. Diam Relat Mater 137:110128. https://doi.org/10.1016/j.diamond.2023.110128
Bundy FP, Hall HT, Strong HM, Wentorfjun RH (1995) Man-made diamonds. Nature 176:51–55. https://doi.org/10.1038/176051a0
Huang M, Tang MQ, Luo XY (2005) Research and development of powder materials for industrial diamond. Powder Metall Ind 15:33–39
Ma Y, Zou G, Yang H, Meng J (1994) Conversion of fullerenes to diamond under high pressure and high temperature. Appl Phys Lett 65:822–823. https://doi.org/10.1063/1.112242
Cao LM, Gao CX, Sun HP, Zou GT, Zhang Z, Zhang XY, He M, Zhang M, Li YC, Zhang J, Dai DY, Sun LL, Wang WK (2001) Synthesis of diamond from carbon nanotubes under high pressure and high temperature. Carbon 39:311–314
Sung CM, Tai MF (1997) Reactivities of transition metals with carbon: implications to the mechanism of diamond synthesis under high pressure. Int J Refract Metal Hard Mater 15:237–256. https://doi.org/10.1016/S0263-4368(97)00003-6
Li Y, Shao H, Lin Z, Lu J, Liu L, Duployer B, Persson POA, Eklund P, Hultman L, Li M, Chen K, Zha XH, Du S, Rozier P, Chai Z, Raymundo-Pinero E, Taberna PL, Simon P, Huang Q (2020) A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat Mater 19:894–899. https://doi.org/10.1038/s41563-020-0657-0
Naguib M, Kurtoglu M, Presser V, Lu J, Niu J, Heon M, Hultman L, Gogotsi Y, Barsoum MW (2011) Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater 23:4248–4253. https://doi.org/10.1002/adma.201102306
Luo R, Li R, Jiang C, Qi R, Liu M, Luo C, Lin H, Huang R, Peng H (2021) Facile synthesis of cobalt modified 2D titanium carbide with enhanced hydrogen evolution performance in alkaline media. Int J Hydrog Energy 46:32536–32545. https://doi.org/10.1016/j.ijhydene.2021.07.110
Jiang H, Gong Q, Peterlechner M (2023) Microstructure analysis of a CoCrFeNi high-entropy alloy after compressive deformation. Mater Sci Eng 888:145785. https://doi.org/10.1016/j.msea.2023.145785
Nath D, Singh F, Das R (2020) X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles-a comparative study. Mater Chem Phys 239:122021. https://doi.org/10.1016/j.matchemphys.2019.122021
Takaki S, Masumura T, Tsuchiyama T (2018) Proposal of simplified modified Williamson-Hall equation. isij Int 58:2354–2356. https://doi.org/10.2355/isijinternational.ISIJINT-2018-517
Tan QY, Yu ZX, Xiang QC, He ND, Long RX, Wang J (2023) Photo-Fenton properties of MIL-88A (Fe)/Ti3C2 MXene with tunable active crystal facets: universal for degradation of common pollutants in wastewater. Process Saf Environ Prot 179:405–420. https://doi.org/10.1016/j.psep.2023.09.020
Peng YH, Zhang Y, Deng LW, Zhang P, Liu YW, Zeng B, Gao XH, Huang SX (2023) Electrostatic self-assembly of Ti3C2Tx/Fe nanochain hybrids for efficient microwave absorber. J Magn Magn Mater 570:170536. https://doi.org/10.1016/j.jmmm.2023.170536
Li N, Xie X, Lu HX, Fan BB, Wang XH, Zhao B, Zhang R, Yang R (2019) Novel two-dimensional Ti3C2Tx/Ni-spheres hybrids with enhanced microwave absorption properties. Ceram Int 45:22880–22888. https://doi.org/10.1016/j.ceramint.2019.07.331
Wen CY, Zhu TJ, Li XY, Li HF, Huang XQ, Sun GB (2020) Nanostructured Ni/Ti3C2Tx MXene hybrid as cathode for lithium-oxygen battery. Chin Chem Lett 31:1000–1003. https://doi.org/10.1016/j.cclet.2019.09.028
Gogotsi Y, Welz S, Ersoy DA, McNallan MJ (2001) Conversion of silicon carbide to crystalline diamond-structured carbon at ambient pressure. Nature 411:283–287. https://doi.org/10.1038/35077031
Gupta SK, Tripathy SP, Bharti D, Pal SK, Verma S, Pal S-K, Ray SS (2023) One pot synthesis of phosphate glass with in situ formed nanodiamonds from adenosine triphosphate for bone repair. Ceram Int 49:22537–22546. https://doi.org/10.1016/j.ceramint.2023.04.089
Han SJ, Seo M, Cho MG, Kwon YW, Lim SH, Han SH (2021) Sub-5 nm nanodiamonds fabricated by plasma immersion ion implantation as fluorescent probes. ACS Appl Nano Mater 4:2238–2246. https://doi.org/10.1021/acsanm.1c00038
Frenklach M, Kematick R, Huang D, Howard W, Spear KE, Phelps AW, Koba R (1989) Homogeneous nucleation of diamond powder in the gas phase. J Appl Phys 66:395–399. https://doi.org/10.1063/1.343890
Sharma AK, Salunke HG, Das GP, Ayyub P, Multani MS (1996) Electronic structure of the 4H polytype of diamond. J Phys Condens Matter 8:5801–5809. https://doi.org/10.1088/0953-8984/8/31/013
Wang JB, Zhang CY, Zhong XL, Yang GW (2002) Cubic and hexagonal structures of diamond nanocrystals formed upon pulsed laser induced liquid–solid interfacial reaction. Chem Phys Lett 361:86–90. https://doi.org/10.1016/S0009-2614(02)00871-0
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Projects 52172052 and 51872253) and Innovation Ability Promotion Program of Hebei (22567609H).
Author information
Authors and Affiliations
Contributions
ZZ: conceptualization and writing-original draft. HM: investigation and formal analysis. JZ: investigation. SL: formal analysis. RZ: writing-reviewing and editing, project administration, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not Applicable.
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Ma, H., Zhang, J. et al. Effect of catalyst type on the type and size of nano-diamond synthesized by chlorine gas etching of Ti3C2 MXene under ambient pressure. J Mater Sci 59, 2814–2827 (2024). https://doi.org/10.1007/s10853-024-09363-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09363-4