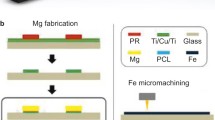
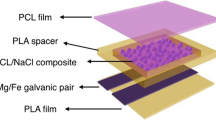
Abstract
The development of biodegradable electronics, or transient electronics, excludes the need for second surgeries for device removal, reduces the potential infection risks, and also opens up opportunities in the development of zero-waste and green electronics which would be degraded after a certain period of operation. Batteries are a vital component of transient electronics since they serve as biodegradable power sources. Recently, magnesium-based biodegradable batteries have gained considerable attention owing to their advantages of high specific capacity, high energy density, long shelf-life, desirable biodegradability, high physiological tolerance, low cost, and high safety. The main drawback of Mg, as a widely explored anode material in biodegradable batteries, is its high degradation rate due to a low corrosion resistance, especially in aqueous environments. Therefore, controlling the degradation rate of Mg anodes is crucial, and this is usually achieved by several strategies such as coating, adding alloying elements, and thermomechanical processing techniques. Moreover, the development of biodegradable battery cathode materials and electrolytes is also crucial for preparing fully biodegradable batteries. The current study is designed to give an overview of the up-to-date research progress on the development of anode, cathode, and electrolyte materials for use in biodegradable Mg batteries. In this respect, the strategies for materials selection, the fabrication schemes, battery architectures, and their electrochemical and in vivo performance are summarized. Finally, the future outlook is discussed to help in the development of green biodegradable Mg batteries that are viable in the fields of medicine, flexible wearables, and consumer electronics.











Similar content being viewed by others
References
Palmroth A, Salpavaara T, Lekkala J, Kellomäki M (2019) Fabrication and characterization of a wireless bioresorbable pressure sensor. Adv Mater Technol 4:3–9. https://doi.org/10.1002/admt.201900428
She D, Allen MG (2020) A self-powered, biodegradable dissolved oxygen microsensor. J Microelectromech Syst 29:1074–1078. https://doi.org/10.1109/JMEMS.2020.3013208
She D, Tsang M, Allen M (2019) Biodegradable batteries with immobilized electrolyte for transient MEMS. Biomed Microdevices 21:1–9. https://doi.org/10.1007/s10544-019-0377-x
Li R, Wang L, Kong D, Yin L (2018) Recent progress on biodegradable materials and transient electronics. Bioact Mater 3:322–333. https://doi.org/10.1016/j.bioactmat.2017.12.001
Huang X, Hou H, Yu B et al (2023) Fully biodegradable and long-term operational primary zinc batteries as power sources for electronic medicine. ACS Nano 17:5727–5739. https://doi.org/10.1021/acsnano.2c12125
Mittal N, Ojanguren A, Kundu D, Lizundia E, Niederberger M (2022) Bottom-up design of a green and transient zinc-ion battery with ultralong lifespan. Small 19:2206249. https://doi.org/10.1002/smll.202206249
Tsang M, Armutlulu A, Martinez AW, Bidstrup Allen SA, Allen MG (2015) Biodegradable magnesium/iron batteries with polycaprolactone encapsulation: a microfabricated power source for transient implantable devices. Microsyst Nanoeng 1:1–10. https://doi.org/10.1038/micronano.2015.24
Lee MH, Lee J, Jung SK et al (2021) A biodegradable secondary battery and its biodegradation mechanism for eco-friendly energy-storage systems. Adv Mater 33:1–11. https://doi.org/10.1002/adma.202004902
Huang X, Wang D, Yuan Z et al (2018) A fully biodegradable battery for self-powered transient implants. Small 14:1–8. https://doi.org/10.1002/smll.201800994
Xia J, Yuan Z, Cai F (2018) Toward a biocompatible and degradable battery using a Mg–Zn–Zr Alloy with β-tricalcium phosphate nanocoating as anode. J Mater Eng Perform 27:4005–4009. https://doi.org/10.1007/s11665-018-3512-6
Stauss S, Honma I (2018) Biocompatible batteries-materials and chemistry, fabrication, applications, and future prospects. Bull Chem Soc Jpn 91:492–505. https://doi.org/10.1246/bcsj.20170325
Shao M, Sheng H, Lin L et al (2023) High-performance biodegradable energy storage devices enabled by heterostructured MoO3–MoS2 composites. Small 19:1–13. https://doi.org/10.1002/smll.202205529
Togonon JJH, Esparcia EA, del Rosario JAD, Ocon JD (2021) Development of magnesium anode-based transient primary batteries. ChemistryOpen 10:471–476. https://doi.org/10.1002/open.202000168
Khan MM, Rahman ZU, Deen KM, Shabib I, Haider W (2020) Sputtered Mg100-xZnx (0 ≤ x ≤ 100) systems as anode materials for a biodegradable battery aimed for transient bioelectronics. Electrochim Acta 329:135129. https://doi.org/10.1016/j.electacta.2019.135129
Tsang M, Armutlulu A, Herrault F et al (2014) Development of electroplated magnesium microstructures for biodegradable devices and energy sources. J Microelectromech Syst 23:1281–1289. https://doi.org/10.1109/JMEMS.2014.2360201
Li D, Yuan Y, Liu J, Fichtner M, Pan F (2020) A review on current anode materials for rechargeable Mg batteries. J Magnes Alloy 8:963–979. https://doi.org/10.1016/j.jma.2020.09.017
Liu F, Cao G, Ban J et al (2022) Recent advances based on Mg anodes and their interfacial modulation in Mg batteries. J Magnes Alloy 10:2699–2716. https://doi.org/10.1016/j.jma.2022.09.004
Tong F, Wei S, Chen X, Gao W (2021) Magnesium alloys as anodes for neutral aqueous magnesium-air batteries. J Magnes Alloy 9:1861–1883. https://doi.org/10.1016/j.jma.2021.04.011
Fu KK, Wang Z, Dai J, Carter M, Hu L (2016) Transient electronics: materials and devices. Chem Mater 28:3527–3539. https://doi.org/10.1021/acs.chemmater.5b04931
Cheng H, Vepachedu V (2016) Recent development of transient electronics. Theor Appl Mech Lett 6:21–31. https://doi.org/10.1016/j.taml.2015.11.012
Han WB, Lee JH, Shin J, Hwang S (2020) Advanced materials and systems for biodegradable, transient electronics. Adv Mater 32:2002211. https://doi.org/10.1002/adma.202002211
Mittal N, Ojanguren A, Niederberger M, Lizundia E (2021) Degradation behavior, biocompatibility, electrochemical performance, and circularity potential of transient batteries. Adv Sci 8:1–26. https://doi.org/10.1002/advs.202004814
Marie J, Abarro E, Ocon JD, Rosario JAD (2022) Evaluation of magnesium-based primary battery for powering transient electronics. Chem Eng Trans 94:151–156. https://doi.org/10.3303/CET2294025
Yun Y, Dong Z, Lee N et al (2009) Revolutionizing biodegradable metals. Mater Today 12:22–32. https://doi.org/10.1016/S1369-7021(09)70273-1
Wei L, Gao Z (2023) Recent research advances on corrosion mechanism and protection, and novel coating materials of magnesium alloys: a review. RSC Adv 13:8427–8463. https://doi.org/10.1039/d2ra07829e
Gao Y, Wang L, Li L, Gu X, Zhang K, Xia J, Fan Y (2019) Effect of stress on corrosion of high-purity magnesium in vitro and in vivo. Acta Biomater 83:477–486. https://doi.org/10.1016/j.actbio.2018.11.019
Sanchez AHM, Luthringer BJC, Feyerabend F, Willumeit R (2015) Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater 13:16–31. https://doi.org/10.1016/j.actbio.2014.11.048
Aghamohammadi H, Hassanzadeh N, Eslami-Farsani R (2021) A review study on the recent advances in developing the heteroatom-doped graphene and porous graphene as superior anode materials for Li–ion batteries. Ceram Int 47:22269–22301. https://doi.org/10.1016/j.ceramint.2021.05.048
Aghamohammadi H, Hassanzadeh N, Eslami-Farsani R (2021) A review study on titanium niobium oxide-based composite anodes for Li–ion batteries: synthesis, structure, and performance. Ceram Int 47:26598–26619. https://doi.org/10.1016/j.ceramint.2021.06.127
Aghamohammadi H, Hassanzadeh N, Eslami-Farsani R (2022) A comprehensive review study on pure titanium niobium oxide as the anode material for Li–ion batteries. J Alloys Compd 911:165117. https://doi.org/10.1016/j.jallcom.2022.165117
Kirkland NT, Staiger MP, Nisbet D, Davies CHJ, Birbilis N (2011) Performance-driven design of biocompatible Mg alloys. Jom 63:28–34. https://doi.org/10.1007/s11837-011-0089-z
Gerashi E, Jamalpour M, Alizadeh R, Labbaf S, Mahmudi R (2023) Effects of hydrothermal coating on the degradation behavior and biocompatibility of an Mg–4Zn–0.3Sr alloy. Mater Lett 330:133224. https://doi.org/10.1016/j.matlet.2022.133224
Mohammadi-zerankeshi M, Zohrevand M, Alizadeh R (2023) Hydrothermal coating of the biodegradable Mg–2Ag alloy. Met 13:1260. https://doi.org/10.3390/met13071260
Mohammadi Zerankeshi M, Alizadeh R, Gerashi E, Asadollahi M, Langdon TG (2022) Effects of heat treatment on the corrosion behavior and mechanical properties of biodegradable Mg alloys. J Magnes Alloy 10:1737–1785. https://doi.org/10.1016/j.jma.2022.04.010
Asadollahi M, Gerashi E, Alizadeh R, Mahmudi R (2022) Effect of Zn content and processing route on the microstructure, mechanical properties, and bio-degradation of Mg–Zn alloys. J Mater Res Technol 21:4473–4489. https://doi.org/10.1016/j.jmrt.2022.11.041
Wang CT, Li Z, Wang JT, Langdon TG (2023) New developments in the processing of metallic alloys for achieving exceptional superplastic properties. Mater Res Proc 32:3–14. https://doi.org/10.21741/9781644902615-1
Abbasi Z, Cabrera JM, Ebrahimi R, Schafler E (2023) Microstructural characteristics, mechanical and corrosion properties of a low-alloyed Mg alloy after different deformation processing. Mater Res Proc 32:197–204. https://doi.org/10.21741/9781644902615-22
Meng B, Pan F, Yang J, Li D, Wan M (2023) Effects of deformation conditions on the superplastic deformation behavior of LZ91 Mg–Li alloy under electric field. Mater Res Proc 32:111–118. https://doi.org/10.21741/9781644902615-12
Zohrevand M, Mohammadi-Zerankeshi M, Nobakht-Farin F, Alizadeh R, Mahmudi R (2022) Degradation behavior of the as-extruded and ECAP-processed Mg–4Zn alloy by Ca addition and hydrothermal coating. J Mater Res Technol 20:1204–1215. https://doi.org/10.1016/j.jmrt.2022.07.072
Gerashi E, Asadollahi M, Alizadeh R, Mahmudi R (2022) Effects of Sr additions on the microstructural stability and mechanical properties of a cast Mg–4Zn alloy. Mater Sci Eng A 843:143127. https://doi.org/10.1016/j.msea.2022.143127
Gerashi E, Alizadeh R, Mahmudi R (2022) Improved corrosion resistance and mechanical properties of biodegradable Mg–4Zn–xSr alloys: effects of heat treatment, Sr additions, and multi-directional forging. J Mater Res Technol 20:3363–3380. https://doi.org/10.1016/j.jmrt.2022.08.072
Mohammadi-Zerankeshi M, Alizadeh R, Labbaf S (2023) Improving mechanical, degradation and biological behavior of biodegradable Mg–2Ag alloy: effects of Y addition and heat treatment. J Mater Res Technol 22:1677–1694. https://doi.org/10.1016/j.jmrt.2022.12.026
Mohammadi Zerankeshi M, Alizadeh R (2022) Ag-incorporated biodegradable Mg alloys. Materialia 23:101445. https://doi.org/10.1016/j.mtla.2022.101445
Gerashi E, Alizadeh R, Langdon TG (2022) Effect of crystallographic texture and twinning on the corrosion behavior of Mg alloys: a review. J Magnes Alloy 10:313–325. https://doi.org/10.1016/j.jma.2021.09.009
Zhu Q, Li Y, Cao F et al (2022) Towards development of a high-strength stainless Mg alloy with Al-assisted growth of passive film. Nat Commun 13:5838. https://doi.org/10.1038/s41467-022-33480-w
Deng M, Wang L, Höche D et al (2021) Approaching “stainless magnesium” by Ca micro-alloying. Mater Horizons 8:589–596. https://doi.org/10.1039/d0mh01380c
Edupuganti V, Solanki R (2016) Fabrication, characterization, and modeling of a biodegradable battery for transient electronics. J Power Sources 336:447–454. https://doi.org/10.1016/j.jpowsour.2016.11.004
Li X, Lu K (2017) Playing with defects in metals. Nat Mater 16:700–701. https://doi.org/10.1038/nmat4929
Hassanzadeh N, Sadrnezhaad SK (2021) Magnetic stirring assisted hydrothermal synthesis of Na3MnCO3PO4 cathode material for sodium-ion battery. Ceram Int 47:26929–26934. https://doi.org/10.1016/j.ceramint.2021.06.104
Hassanzadeh N, Sadrnezhaad SK, Ghorbanzadeh M (2018) An investigation of crystallization kinetics of the Na3MnCO3PO4 cathode material, synthesized by the hydrothermal method. Mater Chem Phys 214:73–79. https://doi.org/10.1016/j.matchemphys.2018.04.070
Wang Z, Fu KK, Liu Z et al (2017) Design of high capacity dissoluble electrodes for all transient batteries. Adv Funct Mater 27:1605724. https://doi.org/10.1002/adfm.201605724
Yin L, Huang X, Xu H, Zhang Y, Lam J, Cheng J, Rogers JA (2014) Materials, designs, and operational characteristics for fully biodegradable primary batteries. Adv Mater 26:3879–3884. https://doi.org/10.1002/adma.201306304
Karami-Mosammam M, Danninger D, Schiller D, Kaltenbrunner M (2022) Stretchable and biodegradable batteries with high energy and power density. Adv Mater 34:1–11. https://doi.org/10.1002/adma.202204457
Jia X, Wang C, Zhao C, Ge Y, Wallace GG (2016) Toward biodegradable Mg-Air bioelectric batteries composed of silk fibroin-polypyrrole film. Adv Funct Mater 26:1454–1462. https://doi.org/10.1002/adfm.201503498
Tran HA, Hoang TT, Maraldo A, Do TN, Kaplan DL, Lim KS, Rnjak-Kovacina J (2023) Emerging silk fibroin materials and their applications: new functionality arising from innovations in silk crosslinking. Mater Today 65:244–259. https://doi.org/10.1016/j.mattod.2023.03.027
Zhou J, Li Y, Xie L et al (2021) Humidity-sensitive, shape-controllable, and transient zinc-ion batteries based on plasticizing gelatin-silk protein electrolytes. Mater Today Energy 21:100712. https://doi.org/10.1016/j.mtener.2021.100712
Pham V, Huy H, So S, Hur J (2021) Inorganic fillers in composite gel polymer electrolytes for high-performance lithium and non-lithium polymer batteries. Nanomater 11:614. https://doi.org/10.3390/nano11030614
Karabelli D, Birke KP, Weeber M (2021) A performance and cost overview of selected solid-state electrolytes: race between polymer electrolytes and inorganic sulfide electrolytes. Batteries 7:18. https://doi.org/10.3390/batteries7010018
Wu F, Zhang K, Liu Y, Gao H, Bai Y, Wang X, Wu C (2020) Polymer electrolytes and interfaces toward solid-state batteries: recent advances and prospects. Energy Storage Mater 33:26–54. https://doi.org/10.1016/j.ensm.2020.08.002
Koliyoor J, Ismayil HS, Sanjeev G, Murari MS (2023) An insight into the suitability of magnesium ion-conducting biodegradable methyl cellulose solid polymer electrolyte film in energy storage devices. J Mater Sci 58:5389–5412. https://doi.org/10.1007/s10853-023-08355-0
Jia X, Yang Y, Wang C et al (2014) Biocompatible ionic liquid-biopolymer electrolyte-enabled thin and compact magnesium-Air batteries. ACS Appl Mater Interfaces 6:21110–21117. https://doi.org/10.1021/am505985z
Shanmuga Priya S, Karthika M, Selvasekarapandian S, Manjuladevi R, Monisha S (2018) Study of biopolymer I-carrageenan with magnesium perchlorate. Ionics (Kiel) 24:3861–3875. https://doi.org/10.1007/s11581-018-2535-1
Koliyoor J, Ismayil HS, Vasachar R, Sanjeev G (2022) Novel solid biopolymer electrolyte based on methyl cellulose with enhanced ion transport properties. J Appl Polym Sci 139:51826. https://doi.org/10.1002/app.51826
Park B, Schaefer JL (2020) Review—polymer electrolytes for magnesium batteries: forging away from analogs of lithium polymer electrolytes and towards the rechargeable magnesium metal polymer battery. J Electrochem Soc 167:070545. https://doi.org/10.1149/1945-7111/ab7c71
Suvarnna K, Shanjitha S, Selvasekarapandian S, Kirubavathy SJ (2023) Investigation of solid bio-membrane based on corn biomass as a proton-conducting bio-electrolyte. Bull Mater Sci 46:112. https://doi.org/10.1007/s12034-023-02946-y
Muthukrishnan M, Shanthi C, Selvasekarapandian S, Premkumar R (2023) Biodegradable flexible proton conducting solid biopolymer membranes based on pectin and ammonium salt for electrochemical applications. Int J Hydrog Energy 48:5387–5401. https://doi.org/10.1016/j.ijhydene.2022.11.152
Shanmuga Priya S, Karthika M, Selvasekarapandian S, Manjuladevi R (2018) Preparation and characterization of polymer electrolyte based on biopolymer I-carrageenan with magnesium nitrate. Solid State Ionics 327:136–149. https://doi.org/10.1016/j.ssi.2018.10.031
Chavan C, Bhajantri RF, Cyriac V, Ismayil SSSB (2023) Investigations on anomalous behavior of ionic conductivity in NaPF6 salt loaded hydroxyethyl cellulose biodegradable polymer electrolyte for energy storage applications. Polym Adv Technol 34:1698–1715. https://doi.org/10.1002/pat.6004
Sangeetha P, Selvakumari TM, Selvasekarapandian S, Mahalakshmi M (2023) Preparation of primary magnesium battery based on kappa carrageenan with magnesium perchlorate and its application to electrochemical devices. Polym Bull. https://doi.org/10.1007/s00289-022-04669-2
Kiruthika S, Malathi M, Selvasekarapandian S, Tamilarasan K, Maheshwari T (2020) Conducting biopolymer electrolyte based on pectin with magnesium chloride salt for magnesium battery application. Polym Bull 77:6299–6317. https://doi.org/10.1007/s00289-019-03071-9
Mahalakshmi M, Selvanayagam S, Selvasekarapandian S, Chandra MVL, Sangeetha P, Manjuladevi R (2020) Magnesium ion-conducting solid polymer electrolyte based on cellulose acetate with magnesium nitrate (Mg(NO3)2·6H2O) for electrochemical studies. Ionics (Kiel) 26:4553–4565. https://doi.org/10.1007/s11581-020-03615-4
Shetty SK, Ismayil SG (2020) Enhancement of electrical and optical properties of sodium bromide doped carboxymethyl cellulose biopolymer electrolyte films. J Macromol Sci Part B 59:235–247. https://doi.org/10.1080/00222348.2020.1711585
Garidepalli T, Parthiban V, Sunita Sundari G, Erothu H (2022) Ionic conductivity studies of biodegradable polymer electrolyte for Mg ion batteries. Asian J Chem 34:1742–1748. https://doi.org/10.14233/ajchem.2022.23683
Jia X, Wang C, Ranganathan V et al (2017) A biodegradable thin-film magnesium primary battery using silk fibroin-ionic liquid polymer electrolyte. ACS Energy Lett 2:831–836. https://doi.org/10.1021/acsenergylett.7b00012
Wang J, Song S, Muchakayala R, Hu X, Liu R (2017) Structural, electrical, and electrochemical properties of PVA-based biodegradable gel polymer electrolyte membranes for Mg-ion battery applications. Ionics (Kiel) 23:1759–1769. https://doi.org/10.1007/s11581-017-1988-y
Author information
Authors and Affiliations
Contributions
NH contributed to conceptualization, writing—original draft, and writing—review and editing. TGL was involved in writing—review and editing.
Corresponding author
Additional information
Handling Editor: C. Barry Carter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassanzadeh, N., Langdon, T.G. Invited viewpoint: biodegradable Mg batteries. J Mater Sci 58, 13721–13743 (2023). https://doi.org/10.1007/s10853-023-08828-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08828-2