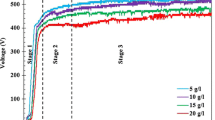
Abstract
By adopting the strategy of generating chelating agents during plasma electrolytic oxidation (PEO), PEO coatings were prepared in acidic eletrolytic solution by constant current mode. The effect of NaF concentration on the coating and the growth and regulation mechanism of fluoride in acidic eletrolytic solution by constant current mode were explored. The composition, structure, corrosion resistance, and formation mechanism of the coating were investigated. The results showed that the addition of NaF into the acidic electrolyte solution caused the pore diameter decreased, while the coating thickness and compactness were increased. The increase of NaF concentration in the acid electrolyte increases the MgF2 content and affects the adhesion of the coating to the substrate, which was attributed to the changes in the spark discharge characteristics. Subsequently, the self-sealing of the coating and the amount of MgF2 composition in the coating will affect the corrosion resistance of the coating. The PEO coating fabricated in the acidic electrolyte solution containing 7 g/L NaF displayed small pore size, stronger bonding, and thicker coating, which resulted in superior corrosion resistance, the lowest corrosion tendency and enhance the polarization resistance.










Similar content being viewed by others
Data availability
Data will be made available on request.
References
Prasad SVS, Prasad SB, Verma K, Mishra RK, Kumar V, Singh S (2022) The role and significance of magnesium in modern day research-a review. J Magnes Alloy 10:1–61. https://doi.org/10.1016/j.jma.2021.05.012
Shao Y, Zeng RC, Li SQ, Cui LY, Zou YH, Guan SK, Zheng YF (2020) Advance in antibacterial magnesium alloys and surface coatings on magnesium alloys: a review. Acta Metall Sin-EngL 33:615–629. https://doi.org/10.1007/s40195-020-01044-w
Li LY, Cui LY, Zeng RC, Li SQ, Chen XB, Zheng Y, Kannan MB (2018) Advances in functionalized polymer coatings on biodegradable magnesium alloys - a review. Acta Biomater 79:23–36. https://doi.org/10.1016/j.actbio.2018.08.030
Yang Y, Xiong XM, Chen J, Peng XD, Chen DL, Pan FS (2021) Research advances in magnesium and magnesium alloys worldwide in 2020. J Magnes Alloy 9:705–747. https://doi.org/10.1016/j.jma.2021.04.001
Zhang Y, Chen F, Zhang Y, Liu ZY, Wang XF, Du C (2019) Influence of graphene oxide on the antiwear and antifriction performance of MAO coating fabricated on MgLi alloy. Surf Coat Technol 364:144–156. https://doi.org/10.1016/j.surfcoat.2019.01.103
Gnedenkov AS, Lamaka SV, Sinebryukhov SL, Mashtalyar DV, Egorkin VS, Imshinetskiy IM, Zheludkevich ML, Gnedenkov SV (2021) Control of the Mg alloy biodegradation via PEO and polymer-containing coatings. Corr Sci 182:109254. https://doi.org/10.1016/j.corsci.2021.109254
Rams J, Torres B, Pulido-González N, García-Rodriguez S (2022) Magnesium alloys: fundamentals and recent advances. Encyclopedia of materials: metals and alloys. Elsevier, pp 2–10. https://doi.org/10.1016/B978-0-12-819726-4.00082-X
Johari NA, Alias J, Zanurin A, Mohamed NS, Alang NA, Zain MZM (2022) Anti-corrosive coatings of magnesium: a review. Mater Today: Proc 48:1842–1848. https://doi.org/10.1016/j.matpr.2021.09.192
Pezzato L, Lorenzetti L, Tonelli L, Bragaggia G, Dabalà M, Martini C, Brunelli K (2021) Effect of SiC and borosilicate glass particles on the corrosion and tribological behavior of AZ91D magnesium alloy after PEO process. Surf Coat Technol 428:127901. https://doi.org/10.1016/j.surfcoat.2021.127901
Zafari A, Ghasemi HM, Mahmudi R (2014) An investigation on the tribological behavior of AZ91 and AZ91+3wt% RE magnesium alloys at elevated temperatures. Mater Design 54:544–552. https://doi.org/10.1016/j.matdes.2013.08.073
Jiang JH, Zhou Q, Yu JS, Ma AB, Song D, Lu FM, Zhang LY, Yang DH, Chen JQ (2013) Comparative analysis for corrosion resistance of micro-arc oxidation coatings on coarse-grained and ultra-fine grained AZ91D Mg alloy. Surf Coat Technol 216:259–266. https://doi.org/10.1016/j.surfcoat.2012.11.055
Barati Darband Gh, Aliofkhazraei M, Hamghalam P, Valizade N (2017) Plasma electrolytic oxidation of magnesium and its alloys: mechanism, properties and applications. J Magnes Alloys 5:74–132. https://doi.org/10.1016/j.jma.2017.02.004
Wen L, Wang YM, Liu Y, Zhou Y, Guo LX, Ouyang JH, Jia DC (2011) EIS study of a self-repairing microarc oxidation coating. Corr Sci 53:618–623. https://doi.org/10.1016/j.corsci.2010.10.010
Yang SK, Sun RX, Chen KZ (2022) Self-healing performance and corrosion resistance of phytic acid/cerium composite coating on microarc-oxidized magnesium alloy. Chem Eng J 428:131198. https://doi.org/10.1016/j.cej.2021.131198
Sampatirao H, Radhakrishnapillai S, Dondapati S, Parfenov E, Nagumothu R (2021) Developments in plasma electrolytic oxidation (PEO) coatings for biodegradable magnesium alloys. Mater Today Proc 46:1407–1415. https://doi.org/10.1016/j.matpr.2021.02.650
Sreekanth D, Rameshbabu N (2012) Development and characterization of MgO/hydroxyapatite composite coating on AZ31 magnesium alloy by plasma electrolytic oxidation coupled with electrophoretic deposition. Mater Lett 68:439–442. https://doi.org/10.1016/j.matlet.2011.11.025
Sun W, Zhang GD, Tan LL, Yang K, Ai HJ (2016) The fluoride coated AZ31B magnesium alloy improves corrosion resistance and stimulates bone formation in rabbit model. Mater Sci Eng C 63:506–511. https://doi.org/10.1016/j.msec.2016.03.016
Panemangalore DB, Shabadi R, Gupta M, Ji G (2019) Effect of fluoride coatings on the corrosion behavior of Mg–Zn–Er alloys. Surf Interfaces 14:72–81. https://doi.org/10.1016/j.surfin.2018.11.007
Yao W, Wu L, Wang J, Jiang B, Zhang D, Serdechnova M, Shulha T, Blawert C, Zheludkevich ML, Pan F (2022) Micro-arc oxidation of magnesium alloys: a review. J Mater Sci Technol 118:158–180. https://doi.org/10.1016/j.jmst.2021.11.053
Liu Y, Zhang Y, Wang YL, Tian YQ, Chen LS (2021) Research progress on surface protective coatings of biomedical degradable magnesium alloys. J Alloys Compd 885:161001. https://doi.org/10.1016/j.jallcom.2021.161001
Rahmati M, Raeissi K, Toroghinejad MR, Hakimizad A, Santamaria M (2022) Corrosion and wear resistance of coatings produced on AZ31 Mg alloy by plasma electrolytic oxidation in silicate-based K2TiF6 containing solution: Effect of waveform. J Magnes Alloys 10:2574–2587. https://doi.org/10.1016/j.jma.2021.07.026
Zoubi WA, Khan ME, Ko YG (2022) A simple method to functionalize the surface of plasma electrolysis produced inorganic coatings for growing different organic structure. Prog Org Coat 171:107008. https://doi.org/10.1016/j.porgcoat.2022.107008
Ma X, Blawert C, Hoeche D, Kainer KU, Zheludkevich ML (2017) A model describing the growth of a PEO coating on AM50 Mg alloy under constant voltage mode. Electrochim Acta 251:461–474. https://doi.org/10.1016/j.electacta.2017.08.147
Ma X, Zhu S, Wang L, Ji C, Ren C, Guan S (2014) Synthesis and properties of a bio-composite coating formed on magnesium alloy by one-step method of micro-arc oxidation. J Alloys Compd 590:247–253. https://doi.org/10.1016/j.jallcom.2013.12.145
Moon S, Kwon D (2016) Anodic oxide films formed on AZ31 magnesium alloy by plasma electrolytic oxidation method in electrolytes containing various NaF concentrations. J Korean Inst Surf Eng 49:225–230. https://doi.org/10.5695/JKISE.2016.49.3.225
Stojadinović S, Vasilić R, Radić-Perić J, Perić M (2015) Characterization of plasma electrolytic oxidation of magnesium alloy AZ31 in alkaline solution containing fluoride. Sur Coat Technol 273:1–11. https://doi.org/10.1016/j.surfcoat.2015.03.032
Gao YL, Jie M, Liu Y (2017) Mechanical properties of Al2O3 ceramic coatings prepared by plasma spraying on magnesium alloy. Surf Coat Technol 315:214–219. https://doi.org/10.1016/j.surfcoat.2017.02.026
Lou BS, Lin YY, Tseng CM, Lu YC, Duh JG, Lee JW (2017) Plasma electrolytic oxidation coatings on AZ31 magnesium alloys with Si3N4 nanoparticle additives. Surf Coat Technol 332:358–367. https://doi.org/10.1016/j.surfcoat.2017.05.094
Fu LX, Yang YX, Zhang LL, Wu YZ, Liang J, Cao BC (2019) Preparation and characterization of fluoride-incorporated plasma electrolytic oxidation coatings on the AZ31 magnesium alloy. Coatings 9:826. https://doi.org/10.3390/coatings9120826
Kazanski B, Kossenko A, Zinigrad M, Lugovskoy A (2013) Fluoride ions as modifiers of the oxide layer produced by plasma electrolytic oxidation on AZ91D magnesium alloy. Appl Surf Sci 287:461–466. https://doi.org/10.1016/j.apsusc.2013.09.180
Shi Y (2020) Study on corrosion properties and microstructure of PEO coatings formed on AZ31 Mg alloy. Int J Electrochem Sci 15:5846–5859. https://doi.org/10.20964/2020.06.39
Wang LQ, Snihirova D, Deng M, Wang C, Vaghefinazari B, Wiese G, Langridge M, Hoeche D, Lamaka SV, Zheludkevich ML (2021) Insight into physical interpretation of high frequency time constant in electrochemical impedance spectra of Mg. Corr Sci 187:109501. https://doi.org/10.1016/j.corsci.2021.109501
Lee KM, Shin KR, Namgung S, Yoo B, Shin DH (2011) Electrochemical response of ZrO2-incorporated oxide layer on AZ91 Mg alloy processed by plasma electrolytic oxidation. Surf Coat Technol 205:3779–3784. https://doi.org/10.1016/j.surfcoat.2011.01.033
Wang LQ, Snihirova D, Deng M, Wang C, Vaghefinazari B, Wiese G, Langridge M, Höche D, Lamaka SV, Zheludkevich ML (2021) Insight into physical interpretation of high frequency time constant in electrochemical impedance spectra of Mg. Corr Sci 187:109501. https://doi.org/10.1016/j.corsci.2021.109501
Wang LQ, Snihirova D, Deng M, Vaghefinazari B, Höche D, Lamaka SV, Zheludkevich ML (2022) Revealing physical interpretation of time constants in electrochemical impedance spectra of Mg via Tribo-EIS measurements. Electrochim Acta 404:139582. https://doi.org/10.1016/j.electacta.2021.139582
Duan HP, Yan CW, Wang FH (2007) Effect of electrolyte additives on performance of plasma electrolytic oxidation films formed on magnesium alloy AZ91D. Electrochim Acta 52:3785–3793. https://doi.org/10.1016/j.electacta.2006.10.066
Liang J, Srinivasan PB, Blawert C, Dietzel W (2009) Comparison of electrochemical corrosion behaviour of MgO and ZrO2 coatings on AM50 magnesium alloy formed by plasma electrolytic oxidation. Corr Sci 51:2483–2492. https://doi.org/10.1016/j.corsci.2009.06.034
Dou Z, Zhang Y, Shulha T, Cui RG, Serdechnova M, Tian H, Yan T, Blawert C, Li L, Zheludkevich ML, Chen F (2022) Insight into chelating agent stimulated in-situ growth of MgAl-LDH films on magnesium alloy AZ31: The effect of initial cationic concentrations. Surf Coat Technol 439:128414. https://doi.org/10.1016/j.surfcoat.2022.128414
Çapraz ÖÖ, Shrotriya P, Skeldon P, Thompson GE, Hebert KR (2015) Role of oxide stress in the initial growth of self-organized porous aluminum oxide. Electrochim Acta 167:404–411. https://doi.org/10.1016/j.electacta.2015.03.017
Zhu LJ, Li HZ, Mei QM, Lu JB, Li ZX (2021) The mechanism for tuning the corrosion resistance and pore density of plasma electrolytic oxidation (PEO) coatings on Mg alloy with fluoride addition. J Magnes Alloys 9:281–291. https://doi.org/10.1016/j.jma.2021.10.007
Dong KH, Song YW, Shan DY, Han EH (2015) Formation mechanism of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Surf Coat Technol 266:188–196. https://doi.org/10.1016/j.surfcoat.2015.02.041
Acknowledgements
This work was supported by the Beijing Natural Science Foundation (2202017), and the URT program of Beijing Institute of Petrochemical Technology (2021J00181 and 2020X00176).
Author information
Authors and Affiliations
Contributions
This article is a joint effort by all members and I will explain their contributions and express my gratitude to them. FC: Conceptualization, Writing—Review and Editing; Funding acquisition. ZR: Writing—Original Draft; Investigation; Fabrication, Validation. YB, LL: Writin—review and editing. Conceptualization. HT: Supervision. ZD: Investigation, Fabrication. YZ: Supervision; Conceptualization.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any products, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Ethical approval
Not applicable.
Additional information
Handling Editor: Catalin Croitoru.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rong, Z., Bai, Y., Tian, H. et al. Influence of sodium fluoride in acidic electrolytic solution on the structure and properties of plasma electrolytic oxidation coating on AZ91D magnesium alloy. J Mater Sci 58, 8103–8117 (2023). https://doi.org/10.1007/s10853-023-08469-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08469-5