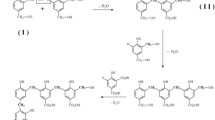
Abstract
Catalytic graphitization has been used since years, normally by heating from room temperature to 2227 or 3027 °C. Ferrocene was used to induce the graphitization in modified novolak phenolic resins synthesized in laboratory (PR). In this study, the intermediate carbon structures containing iron during the graphitization process, obtained after the different steps of heat treatment from 200 to 1000 °C, were identified concerning the oxidation states of iron and morphological and structural variations. The role of iron in these intermediate structures has not been fully evaluated yet by resonance and spectroscopy techniques; therefore, in this study, it will be discussed briefly. The following techniques were employed: X-ray powder diffraction (XRD), high-resolution transmission electron microscopy (HR-TEM), standard solid-state 13carbon nuclear magnetic resonance (solid-state 13C-NMR), 57Fe-Mössbauer and electron paramagnetic resonance (EPR) spectroscopies. In the material (PRFc) obtained by heat treatment for 5 h at 1000 °C, there were identified Fe2O3 nanocrystals, as well as Fe2O3, Fe3C and γ-iron present inside and outside of an onion-like hollow carbon structure. This structure of PRFc treated at 1000 °C has shown high efficiency in removing the pesticide atrazine (ATZ) in an aqueous medium and influenced the degradation mechanism of ATZ and the formation of atrazine-2-hydroxy (HAT).
Graphical abstract










Similar content being viewed by others
References
Sengupta R, Bhattacharya M, Bandyopadhyay S, Bhowmick AK (2011) A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog Polym Sci 36:638–670. https://doi.org/10.1016/j.progpolymsci.2010.11.003
Stamatin I, Morozan A, Dumitru A, Ciupina V, Prodan G, Niewolski J, Figiel H (2007) The synthesis of multi-walled carbon nanotubes (MWNTs) by catalytic pyrolysis of the phenol-formaldehyde resins. Phys E 37:44–48. https://doi.org/10.1016/j.physe.2006.10.013
Chen L, Hernandez Y, Feng X, Mullen K (2012) From nanographene and graphene nanoribbons to graphene sheets: chemical synthesis. Angew Chem Int Ed 51:2–17. https://doi.org/10.1002/anie.201201084
Sierra U, Alvarez P, Blanco C, Granda M, Santamaria R, Menendez R (2015) New alternatives to graphite for producing graphene materials. Carbon 93:812–818. https://doi.org/10.1016/j.carbon.2015.05.105
Fadlalla MI, Kumar PS, Selvam V, Babu SG (2020) Emerging energy and environmental application of graphene and their composites: a review. J Mater Sci 55:7156–7183. https://doi.org/10.1007/s10853-020-04474-0
Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S (2011) Graphene based materials : past, present and future. Prog Mater Sci 56:1178–1271. https://doi.org/10.1016/j.pmatsci.2011.03.003
Jariwala D, Sangwan VK, Lauhon LJ, Marks TJ, Hersam MC (2013) Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem Soc Rev 42:2824–2860. https://doi.org/10.1039/c2cs35335k
Siokou A, Ravani F, Karakalos S, Frank O, Kalbac M, Galiotis C (2011) Surface refinement and electronic properties of graphene layers grown on copper substrate: an XPS, UPS and EELS study. Appl Surf Sci 257(2011):9785–9790. https://doi.org/10.1016/j.apsusc.2011.06.017
Ozaki J, Nozawa K, Yamada K, Uchiyama Y, Yoshimoto Y, Furuichi A, Yokoyama T, Oya A et al (2006) Structures, physicochemical properties and oxygen reduction activities of carbons derived from ferrocene-poly(furfuryl alcohol) mixtures. J Appl Electrochem 36:239–247. https://doi.org/10.1007/s10800-005-9054-2
Talapatra S, Ganesan PG, Kim T, Vajtai R, Huang M, Shima M, Ramanath G, Srivastava D et al (2005) Irradiation-induced magnetism in carbon nanostructures. Phys Rev Lett 95:097201–097204. https://doi.org/10.1103/PhysRevLett.95.097201
Lehtinen PO, Foster AS, Ma Y, Krasheninnikov AV, Nieminen RM (2004) Irradiation-induced magnetism in graphite: a density functional study. Phys Rev Lett 93:187202–187204. https://doi.org/10.1103/PhysRevLett.93.187202
Graphitization heat treatment, IUPAC Compendium of Chemical Terminology. Gold B. (n.d.). https://goldbook.iupac.org/G02692.html. Accessed 2 Mar 2020
Rand B, McEnaney B (1985) Carbon binders from polymeric resins and pitch, Part I-Pyrolysis behaviour and structure of the carbons. Br Ceram Trans J 84:157–165. https://www.researchgate.net/publication/279769595_Carbon_Binders_from_Polymeric_Resins_and_Pitch_Part_I_Pyrolisis_Behaviour_and_Structure_of_the_Carbons. Accessed 2 Mar 2020
Wang Y, Wang S, Bian C, Zhong Y, Jing X (2015) Effect of chemical structure and cross-link density on the heat resistance of phenolic resin. Polym Degrad Stab 111:239–246. https://doi.org/10.1016/j.polymdegradstab.2014.11.016
Renda CG, Bertholdo R (2018) Study of phenolic resin and their tendency for carbon graphitization. J Polym Res 25:241. https://doi.org/10.1007/s10965-018-1635-y
Oya A, Marsh H (1982) Phenomena of catalytic graphitization. J Mater Sci 17:309–322. https://doi.org/10.1007/BF00591464
Acheson EG, Manufacture of Graphite, US568,323, 1896. https://patents.google.com/patent/US568323A/en. Accessed 2 Mar 2020
Acheson EG, Method of Manufacturing Graphite, US645285, 1900. https://patentimages.storage.googleapis.com/f8/61/d5/d6306dafd2e398/US645285.pdf. Accessed 2 Mar 2020
Oya A, Otani S (1979) Catalytic graphitization of carbons by various metals. Carbon 17:131–137. https://doi.org/10.1016/0008-6223(79)90020-4
Luz AP, Renda CG, Lucas AA, Bertholdo R, Aneziris CG, Pandolfelli VC (2017) Graphitization of phenolic resins for carbon-based refractories. Ceram Int 43:8171–8182. https://doi.org/10.1016/j.ceramint.2017.03.143
Renda CG, Bertholdo R, Venâncio T, Luz AP, Pandolfelli VC, Lucas AA (2019) Influence of the mixing process on the graphitization of phenolic resins. Ceram Int 45:12196–12204. https://doi.org/10.1016/j.ceramint.2019.03.124
Omori M, Nagashima K, Yajima S (1977) Synthesis of ferrocene-phenol resins and liberation of iron particles by pyrolysis. Bull Chem Soc Jpn 50:1157–1160. https://doi.org/10.1246/bcsj.50.1157
Bitencourt CS, Luz AP, Pagliosa C, Pandolfelli VC (2015) Role of catalytic agents and processing parameters in the graphitization process of a carbon-based refractory binder. Ceram Int 41:13320–13330. https://doi.org/10.1016/j.ceramint.2015.07.115
Rastegar H, Bavand-vandchali M, Nemati A, Golestani-Fard F (2018) Catalytic graphitization behavior of phenolic resins by addition of in situ formed nano-Fe particles. Phys E Low Dimens Syst Nanostruct 101:50–61. https://doi.org/10.1016/j.physe.2018.03.013
Zhao M, Song H (2011) Catalytic graphitization of phenolic resin. J Mater Sci Technol 27:266–270. https://doi.org/10.1016/S1005-0302(11)60060-1
Yun J, Chen L, Zhang X, Zhao H, Wen Z, Zhang C (2017) The effects of silicon and ferrocene on the char formation of modified novolac resin with high char yield. Polym Degrad Stab 139:97–106. https://doi.org/10.1016/j.polymdegradstab.2017.03.018
Das B, Kusz J, Reddy VR, Zubko M, Bhattacharjee A (2017) Solventless synthesis, morphology, structure and magnetic properties of iron oxide nanoparticles. Solid State Sci 74:62–69. https://doi.org/10.1016/j.solidstatesciences.2017.10.010
Odularu AT (2018) Metal nanoparticles: thermal decomposition, biomedicinal applications to cancer treatment, and future perspectives. Bioinorg Chem Appl. https://doi.org/10.1155/2018/9354708
Bystrzejewski M, Klingeler R, Gemming T, Büchner B, Rümmeli MH (2011) Synthesis of carbon-encapsulated iron nanoparticles by pyrolysis of iron citrate and poly(vinyl alcohol): a critical evaluation of yield and selectivity. Nanotechnology 22:315606. https://doi.org/10.1088/0957-4484/22/31/315606
Park CM, Kim YM, Kim K, Wang D, Su C, Yoon Y (2019) Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: a mini review. Chemosphere 221:392–402. https://doi.org/10.1016/j.chemosphere.2019.01.063
Pastrana-Martínez LM, Morales-Torres S, Figueiredo JL, Faria JL, Silva AMT (2015) Graphene oxide based ultrafiltration membranes for photocatalytic degradation of organic pollutants in salty water. Water Res 77:179–190. https://doi.org/10.1016/j.watres.2015.03.014
Lazarević-Pašti T, Anićijević V, Baljozović M, Anićijević DV, Gutić S, Vasić V, Skorodumova NV, Pašti IA (2018) The impact of the structure of graphene-based materials on the removal of organophosphorus pesticides from water. Environ Sci Nano 5:1482–1494. https://doi.org/10.1039/c8en00171e
Rambabu N, Guzman CA, Soltan J, Himabindu V (2012) Adsorption characteristics of atrazine on granulated activated carbon and carbon nanotubes. Chem Eng Technol 35:272–280. https://doi.org/10.1002/ceat.201100376
Moreira AJ, Pinheiro BS, Araújo AF, Freschi GPG (2016) Evaluation of atrazine degradation applied to different energy system. Environm Sci Pollut Res 23:18502–18511. https://doi.org/10.1007/s11356-016-6831-x
Fernández-Domene RM, Sánchez-Tovar R, Lucas-granados B, Muñoz-Portero MJ, García-Antón J (2018) Elimination of pesticide atrazine by photoelectrocatalysis using a photoanode based on WO3 nanosheets. Chem Eng J 350:1114–1124. https://doi.org/10.1016/j.cej.2018.06.015
Moreira AJ, Borges AC, Gouvea LFC, MacLeod TCO, Freschi GPG (2017) The process of atrazine degradation, its mechanism, and the formation of metabolites using UV and UV/MW photolysis. J Photochem Photobiol A Chem 347:160–167. https://doi.org/10.1016/j.jphotochem.2017.07.022
Klementová Š, Hornychová L, Šorf M, Zemanová J, Kahoun D (2019) Toxicity of atrazine and the products of its homogeneous photocatalytic degradation on the aquatic organisms Lemna minor and Daphnia magna. Environ Sci Pollut Res 26:27259–27267. https://doi.org/10.1007/s11356-019-05710-0
Moreira AJ, Borges AC, Sousa BB, Mendonça VR, Freschi CD, Freschi GPG (2019) Photodegradation of fluoxetine applying different photolytic reactors: evaluation of the process efficiency and mechanism. J Braz Chem Soc 30:1010–1024. https://doi.org/10.21577/0103-5053.20180250
Hatfield GR, Maciel GE (1987) Solid-state NMR study of the hexamethylenetetramine curing of phenolic resins. Macromolecules 20:608–615. https://doi.org/10.1021/ma00169a024
Shantya A, Ravindran TR, Philip J (2018) Superior thermal stability of polymer capped Fe3O4 magnetic nanoclusters. J Am Ceram Soc 101:483–491. https://doi.org/10.1111/ijlh.12426
Idesaki A, Yamamoto S, Sugimoto M, Yamaki T, Maekawa Y (2020) Formation of Fe nanoparticles by ion implantation technique for catalytic graphitization of a phenolic resin. Quantum Beam Sci 4:1–11. https://doi.org/10.3390/qubs4010011
Gong Z, Bai C, Qiang L, Gao K, Zhang J, Zhang B (2018) Onion-like carbon films endow macro-scale superlubricity. Diam Relat Mater 87:172–176. https://doi.org/10.1016/j.diamond.2018.06.004
Zhang C, Li J, Liu E, He C, Shi C, Du X, Hauge RH, Zhao N (2012) Synthesis of hollow carbon nano-onions and their use for electrochemical hydrogen storage. Carbon 50:3513–3521. https://doi.org/10.1016/j.carbon.2012.03.019
McDonough JK, Frolov AI, Presser V, Niu J, Miller CH, Ubieto T, Fedorov MV, Gogotsi Y (2012) Influence of the structure of carbon onions on their electrochemical performance in supercapacitor electrodes. Carbon 50:3298–3309. https://doi.org/10.1016/j.carbon.2011.12.022
Raghu AV, Karuppanan KK, Pullithadathil B (2019) Highly surface active phosphorus-doped onion-like carbon nanostructures: ultrasensitive, fully reversible, and portable NH3 gas sensors. ACS Appl Electron Mater 1:2208–2219. https://doi.org/10.1021/acsaelm.9b00412
Hung CM, Hoa ND, Duy NV, Toan NV, Le DTT, Hieu NV (2016) Synthesis and gas-sensing characteristics of α-Fe2O3 hollow balls. J Sci Adv Mater Devices 1:45–50. https://doi.org/10.1016/j.jsamd.2016.03.003
Gao R, Zhou S, Chen M, Wu L (2011) Facile synthesis of monodisperse meso-microporous Ta3N5 hollow spheres and their visible light-driven photocatalytic activity. J Mater Chem 21:17087–17090. https://doi.org/10.1039/c1jm13756e
Wang D, Hu J, Yang J, Xiao K, Liang S, Xu J, Liu B, Hou H (2020) Fe and N co-doped carbon derived from melanine resin capsuled biomass as efficient oxygen reduction catalyst for air-cathode microbial fuel cells. Int J Hydrogen Energy 45:3163–3175. https://doi.org/10.1016/j.ijhydene.2019.11.201
Santoro C, Serov A, Stariha L, Kodali M, Gordon J, Babanova S, Bretschger O, Artyushkova K, Atanassov P (2016) Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ Sci 9:2346–2353. https://doi.org/10.1039/c6ee01145d
Dar MI, Shivashankar SA (2014) Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. R Soc Chem 4:4105–4113. https://doi.org/10.1039/c3ra45457f
Wu W, Xiao XH, Zhang SF, Peng TC, Zhou J, Ren F, Jiang CZ (2010) Synthesis and magnetic properties of maghemite (γ-Fe2O3) short-nanotubes. Nanoscale Res Lett 5:1474–1479. https://doi.org/10.1007/s11671-010-9664-4
Surowiec Z, Miaskowski A, Budzyński M (2017) Investigation of magnetite Fe3O4 nanoparticles for magnetic hyperthermia. Nukleonika 62:183–186. https://doi.org/10.1515/nuka-2017-0028
Dézsi I, Fetzer C, Gombkötö Á, Szucs JG, Ungár T (2008) Phase transition in nanomagnetite. J Appl Phys 103:104312. https://doi.org/10.1063/1.2937252
Bhattacharjee A, Rooj A, Roy M, Kusz J, Gutlich P (2013) Solventless synthesis of hematite nanoparticles using ferrocene. J Mater Sci 48:2961–2968. https://doi.org/10.1007/s10853-012-7067-x
Da Costa GM, De Grave E, Bowen LH, Vanderberghe RE, De Bakker PMA (1994) The center shift in mossbauer spectra of maghemite and aluminum maghemites. Clays Clay Miner 42:628–633. https://doi.org/10.1346/CCMN.1994.0420515
Kniep B, Constantinescu A, Niemeier D, Becker KD (2003) An in-situ Mössbauer study of the formation of cementite, Fe3C. Z Fur Anorg Und Allg Chem 629:1795–1804. https://doi.org/10.1002/zaac.200300136
Scrimshire A, Lobera AL, Kultyshev R, Ellis P, Forder SD, Bingham PA (2015) Variable temperature 57Fe-mössbauer spectroscopy study of nanoparticle iron carbides. Croat Chem Acta 88:531–537. https://doi.org/10.5562/cca2782
Wertheim GK, Herber RH (1963) Fe57 mössbauer effect in ferrocene derivatives. J Chem Phys 38:2106–2111. https://doi.org/10.1063/1.1733940
Gonser U, Meechan CJ, Muir AH, Wiedersich H (1963) Determination of néel temperatures in fcc iron. J Appl Phys 34:2373–2378. https://doi.org/10.1063/1.1702749
Wang Y-G, Korai Y, Mochida I, Nagayama K, Hatano H, Fukuda N (2001) Modification of synthetic mesophase pitch with iron oxide, Fe2O3. Carbon 39:1627–1634. https://doi.org/10.1016/S0008-6223(00)00281-5
Bitencourt CS (2011) Cerâmicas Refratárias Resinadas: Fundamentos, Análise Crítica e Efeito dos Agentes Gratifizantes e Antioxidantes, PPGCEM-UFSCAR. https://repositorio.ufscar.br/handle/ufscar/848. Accessed 2 Mar 2020
Wei G, Zhu B, Li X, Ma Z (2015) Microstructure and mechanical properties of low-carbon MgO–C refractories bonded by an Fe nanosheet-modified phenol resin. Ceram Int 41:1553–1566. https://doi.org/10.1016/j.ceramint.2014.09.091
Souza NS, Sergeenkov S, Rodrigues AD, Cardoso CA, Pardo H, Faccio R, Mombrú AW, Galzerani JC et al (2013) Stability issues and structure-sensitive magnetic properties of nanofluid ferromagnetic graphite. J Nanofluids 1:143–147. https://doi.org/10.1166/jon.2012.1022
Kumar RV, Coto M (2018) Visible-light-active photocatalysis: nanostructured catalyst design, mechanisms, and applications. In: Ghosh S (ed) Visible-light-active photocatalysis. Wiley, Weinheim, pp 499–526. https://doi.org/10.1002/9783527808175.ch18
Acknowledgement
The authors would like to express their gratitude to Magnesita Refratários SA for the materials used in this study and the Federation for International Refractory Research and Education (FIRE), to the Laboratory of Nuclear Magnetic Resonance, Department of Chemistry-UFSCar, DEMa-UFSCar, Department of Ceramics-UFSCar, Federal University of Alfenas, Institute of Science and Technology, University of São Paulo, São Carlos Institute of Physics, CBPF-Brazilian Center for Research in Physics, Laboratory of Structural Characterization (LCE-DEMa/UFSCar) and LAMAV-UFSCar for their technical assistance in this study. In addition, E. Baggio Saitovitch acknowledges support from FAPERJ through several grants including Emeritus Professor Fellowship and Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brasil (CNPq) for BPA and corresponding grants. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001. The authors thank the Federal University of Sao Carlos, Post Graduate Program in Materials Science and Engineering (PPGCEM) and CNPq (Process 168691/2017-5) for the financial assistance in disseminating this research.
Funding
The corresponding author (Carmen Greice Renda) was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brasil-CNPq (Grant Number 168691/2017-5) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Dale Huber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Renda, C.G., Contreras Medrano, C.P., Costa, L.J.D. et al. Role of ferrocene-derived iron species in the catalytic graphitization of novolak resins. J Mater Sci 56, 1298–1311 (2021). https://doi.org/10.1007/s10853-020-05312-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05312-z