Abstract
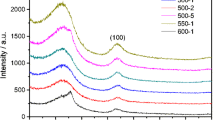
Sodium-ion batteries are regarded as the most promising alternative candidates for lithium-ion batteries. Hard carbon, as a kind of anode materials, has been demonstrated to deliver high specific capacity and stable cycling performance. However, it is still difficult to strike the balance between the relatively high cost and the superior electrochemical performance. We successfully fabricated low-cost lignite-derived hard carbons (a-LCs) with easy scale-up method. The microstructure, morphology and surface information of the obtained a-LCs are evaluated by X-ray diffraction, Raman spectrum and Fourier transform infrared spectrometer. By changing carbonization temperature, a-LCs’ microstructure and defect composition can be tuned and quite different sodium-ion storage behaviors can be seen. When carbonization temperature increases, the carbon microcrystallites grow and defects decay, resulting in a decrease in defect absorption capacity and an increase in graphitic-like nanodomain intercalation capacity. Particularly, a-LC carbonized at 1200 °C (a-LC-1200) can deliver a high capacity of 256 mAh g−1 with the initial Coulombic efficiency of 82%. Besides, it also exhibits a superior rate performance of 210, 197, 180, 168 and 146 mAh g−1 at current rates of 1, 2, 5, 10 and 20C (defined that 1C = 200 mA g−1), respectively. It solves the above problems very well and displays great commercial value.








Similar content being viewed by others
References
Scrosati B, Hassoun J, Sun YK (2011) Lithium-ion batteries. A look into the future. Energy Environ Sci 4:3287–3395
Li Y, Lu Y, Zhao C, Hu YS, Titirici MM, Li H, Huang X, Chen L (2017) Recent advances of electrode materials for low-cost sodium-ion batteries towards practical application for grid energy storage. Energy Storage Mater 7:130–151
Kim H, Kim H, Ding Z, Lee MH, Lim K, Yoon G, Kang K (2016) Recent progress in electrode materials for sodium-ion batteries. Adv Energy Mater 6:1600943. https://doi.org/10.1002/aenm.201600943
Eftekhari A, Jian ZL, Ji XL (2017) Potassium secondary batteries. ACS Appl Mater Interfaces 9:4404–4419
Hwang JY, Myung ST, Sun YK (2017) Sodium-ion batteries: present and future. Chem Soc Rev 46:3529–3614
Liu Q, Hu Z, Chen M, Zou C, Jin H, Wang S, Chou SL, Dou SX (2019) Recent progress of layered transition metal oxide cathodes for sodium-ion batteries. Small. https://doi.org/10.1002/smll.201805381
Chen SQ, Wu C, Shen LF, Zhu CB, Huang YY, Xi K, Maier J, Yu Y (2017) Challenges and perspectives for NASICON-type electrode materials for advanced sodium-ion batteries. Adv Mater. https://doi.org/10.1002/adma.201700431
Guo SP, Li JC, Xu QT, Ma Z, Xue HG (2017) Recent achievements on polyanion-type compounds for sodium-ion batteries: syntheses, crystal chemistry and electrochemical performance. J Power Sources 361:285–299
Fouletier PGM (1988) Electrochemical intercalation of sodium in graphite. Solid State lon 28:1172–1175
Luo W, Jian ZL, Xing ZY, Wang W, Bommier C, Lerner MM, Ji XL (2015) Electrochemically expandable soft carbon as anodes for Na-ion batteries. ACS Cent Sci 1:516–522
Xiao L, Cao Y, Henderson WA, Sushko ML, Shao Y, Xiao J, Wang W, Engelhard MH, Nie Z, Liu J (2016) Hard carbon nanoparticles as high-capacity, high-stability anodic materials for Na-ion batteries. Nano Energy 19:279–288
Zhang HW, Hu MX, Lv Q, Yang L, Lv RT (2019) Monodisperse nitrogen-doped carbon spheres with superior rate capacities for lithium/sodium ion storage. Electrochim Acta 297:365–371
Li ZF, Jian ZL, Wang XF, Rodriguez-Perez IA, Bommier C, Ji XL (2017) Hard carbon anodes of sodium-ion batteries: undervalued rate capability. Chem Commun 53:2610–2613
Yamamoto H, Muratsubaki S, Kubota K, Fukunishi M, Watanabe H, Kim JM, Komaba S (2018) Synthesizing higher-capacity hard-carbons from cellulose for Na- and K-ion batteries. J Mater Chem A 6:16844–16848
Liu RF, Li YL, Wang CL, Xiao N, He L, Guo HY, Wan P, Zhou Y, Qiu JS (2018) Enhanced electrochemical performances of coal liquefaction residue derived hard carbon coated by graphene as anode materials for sodium-ion batteries. Fuel Process Technol 178:35–40
Zhang YJ, Li X, Dong P, Wu G, Xiao J, Zeng XY, Zhang YJ, Sun XL (2018) Honeycomb-like hard carbon derived from pine pollen as high-performance anode material for sodium-ion battery. ACS Appl Mater Interfaces 10:42796–42803
Luo W, Bommier C, Jian ZL, Li X, Carter R, Vail S, Lu Y, Lee JJ, Ji XL (2015) Low-surface-area hard carbon anode for Na-ion batteries via graphene oxide as a dehydration agent. ACS Appl Mater Interfaces 7:2626–2631
Li ZF, Bommier C, Chong ZS, Jian ZL, Surta TW, Wang X, Xing Z, Neuefeind JC, Stickle WF, Dolgos M, Greaney PA, Ji XL (2017) Mechanism of Na-ion storage in hard carbon anodes revealed by heteroatom doping. Adv Energy Mater 7:1602894. https://doi.org/10.1002/aenm.201602894
Komaba S, Murata W, Ishikawa T, Yabuuchi N, Ozeki T, Nakayama T, Ogata A, Gotoh K, Fujiwara K (2011) Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-ion batteries. Adv Funct Mater 21:3859–3867
Zhuang ZH, Cui YL, Zhu HG, Shi YL, Zhuang QC (2018) Coal-based amorphous carbon as economical anode material for sodium-ion battery. J Electrochem Soc 165:A2225–A2232
Zhu ZY, Li F, Zhou ZR, Zeng XY, Wang D, Dong P, Zhao JB, Sun SG, Zhang YJ, Li X (2018) Expanded biomass-derived hard carbon with ultrastable performance in sodium-ion batteries. J Mater Chem A 6:1513–1522
Sadezky A, Muckenhuber H, Grothe H, Niessner R, Pöschl U (2005) Raman microspectroscopy of soot and related carbonaceous materials: spectral analysis and structural information. Carbon 43:1731–1742
Xi YB, Yang DJ, Qiu XQ, Wang H, Huang JH, Li Q (2018) Renewable lignin-based carbon with a remarkable electrochemical performance from potassium compound activation. Ind Crop Prod 124:747–754
Zheng ZJ, Su Q, Zhang Q, Ye H, Wang ZB (2018) Onion-like carbon microspheres as long-life anodes materials for Na-ion batteries. J Mater Sci 53:12421–12431. https://doi.org/10.1007/s10853-018-2515-x
Xu SD, Zhao Y, Liu SB, Ren XX, Chen L, Shi WJ, Wang XM, Zhang D (2018) Curly hard carbon derived from pistachio shells as high-performance anode materials for sodium-ion batteries. J Mater Sci 53:12334–12351. https://doi.org/10.1007/s10853-018-2472-4
Qiu S, Xiao LF, Sushko ML, Han KS, Shao YY, Yan MY, Liang XM, Mai LQ, Feng JW, Cao YL, Ai XP, Yang HX, Liu J (2017) Manipulating adsorption-insertion mechanisms in nanostructured carbon materials for high-efficiency sodium ion storage. Adv Energy Mater 7:1700403. https://doi.org/10.1002/aenm.201700403
Xiao LF, Lu HY, Fang YJ, Sushko ML, Cao YL, Ai XP, Yang HX, Liu J (2018) Low-defect and low-porosity hard carbon with high Coulombic efficiency and high capacity for practical sodium ion battery anode. Adv Energy Mater 8:1703238. https://doi.org/10.1002/aenm.201703238
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51972258) and the Fundamental Research Funds for the Central Universities (WUT: 2019IVA007).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zou, Y., Li, H., Qin, K. et al. Low-cost lignite-derived hard carbon for high-performance sodium-ion storage. J Mater Sci 55, 5994–6004 (2020). https://doi.org/10.1007/s10853-020-04420-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04420-0