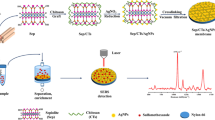
Abstract
A disposable solid-phase extraction (SPE) membrane was developed for rapid removal and sensitive surface-enhanced Raman scattering (SERS) detection of antibiotics in water samples. The membrane was fabricated on commercially available SPE column by filtration of the activated carbon modified with silver nanoparticles (Ag NPs/AC). The prepared SPE membrane exhibited outstanding preconcentration ability due to the high adsorption properties of AC, and excellent ability to enhance Raman signal resulting from “hot spots” between the embedded Ag NPs, improving the sensitivity of SERS detection. A detection limit (LOD) of 5.0 × 10−11 and 1.6 × 10−10 M was achieved for rhodamine 6G and p-aminothiophenol. In addition, the membrane exhibited high reproducibility with spot-to-spot variation in SERS spectral intensity less than 15%. Based on the membrane, the qualitative and quantitative analysis of the antibiotics in aqueous solution was accomplished with the LOD at nM level, demonstrating the feasibility of the disposable SPE membrane for in situ rapid preconcentration and detection.





Similar content being viewed by others
References
Martens E, Demain AL (2011) Platensimycin and platencin: promising antibiotics for future application in human medicine. J Antibiot 43(11):705–710
Yang JH, Bhargava P, McCloskey D, Mao N, Palsson BO, Collins JJ (2017) Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function. Cell Host Microbe 22(6):757-765.e3
Faten F, Azzazy HME, Niessen WMA (2015) Challenges in the determination of aminoglycoside antibiotics: a review. Anal Chim Acta 890(26):21–43
Thorsten C, Schneider RJ, Färber HA, Dirk S, Meyer MT, Goldbach HE (2010) Determination of antibiotic residues in manure, soil, and surface waters. CLEAN Soil Air Water 31(1):36–44
Zhou JL, Kang Y (2013) Matrix effect in high-performance liquid chromatography-tandem mass spectrometry analysis of antibiotics in environmental water samples. J Sep Sci 36(3):564–571
Cazorlareyes R, Romerogonzález R, Frenich AG, Rodríguez Maresca MA, Martínez Vidal JL (2014) Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 89(4):203–212
Moreno-González D, Hamed AM, Gilbert-López B, Gámiz-Gracia L, García-Campaña AM (2017) Evaluation of a multiresidue capillary electrophoresis-quadrupole-time-of-flight mass spectrometry method for the determination of antibiotics in milk samples. J Chromatogr A 1510:100–107
Preu M, Guyot D, Petz M (1998) Development of a gas chromatography-mass spectrometry method for the analysis of aminoglycoside antibiotics using experimental design for the optimisation of the derivatisation reactions. J Chromatogr A 818(1):95–108
Lan L, Yao Y, Ping J, Ying Y (2017) Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens Bioelectron 91:504–514
Adrian J, Pinacho DG, Granier B, Diserens JM, Sãn BF, Marco MP (2008) A multianalyte ELISA for immunochemical screening of sulfonamide, fluoroquinolone and ss-lactam antibiotics in milk samples using class-selective bioreceptors. Anal Bioanal Chem 391(5):1703–1712
Li YT, Qu LL, Li DW, Song QX, Fathi F, Long YT (2013) Rapid and sensitive in situ detection of polar antibiotics in water using a disposable Ag–graphene sensor based on electrophoretic preconcentration and surface-enhanced Raman spectroscopy. Biosens Bioelectron 43(10):94–100
Cacciatore G, Petz M, Rachid S, Hakenbeck R, Bergwerff AA (2004) Development of an optical biosensor assay for detection of B-lactam antibiotics in milk using the penicillin-binding protein 2x*. Anal Chim Acta 520(1):105–115
Xu KC, Yan HP, Tan CF, Lu YY, Li Y, Ho GW, Ji R, Hong M (2018) Hedgehog inspired CuO nanowires/Cu2O composites for broadband visible-light-driven recyclable surface enhanced Raman scattering. Adv Opt Mater 6(7):1701167
Qu LL, Li DW, Xue JQ, Zhai WL, Fossey JS, Long YT (2012) Batch fabrication of disposable screen printed SERS arrays. Lab Chip 12(5):876–881
Cherukulappurath S, Si HL, Campos A, Haynes CL, Oh SH (2014) Rapid and sensitive in situ SERS detection using dielectrophoresis. Chem Mater 26(7):2445–2452
Carrasco S, Benitopena E, Navarrovilloslada F, Langer J, Sanzortiz MN, Reguera J, Lizmarzan LM, Morenobondi MC (2016) Multi-branched gold-mesoporous silica nanoparticles coated with molecularly imprinted polymer for label-free antibiotic SERS analysis. Chem Mater 28(21):7947–7954
Holthoff EL, Stratis-Cullum DN, Hankus ME (2011) A nanosensor for TNT detection based on molecularly imprinted polymers and surface enhanced Raman scattering. Sensors 11(3):2700–2714
Li D, Duan HZ, Wang YH, Zhang QM, Cao HR, Deng W, Li DW (2018) On-site preconcentration of pesticide residues in a drop of seawater by using electrokinetic trapping, and their determination by surface-enhanced Raman scattering. Microchim Acta 185(1):10
Torul H, Çiftçi H, Çetin D, Suludere Z, Boyacı IH, Tamer U (2015) Paper membrane-based SERS platform for the determination of glucose in blood samples. Anal Bioanal Chem 407(27):8243–8251
Balakrishnan VK, Terry KA, Toito J (2006) Determination of sulfonamide antibiotics in wastewater: a comparison of solid phase microextraction and solid phase extraction methods. J Chromatogr A 1131(1–2):1–10
Lai Y, Cui J, Jiang X, Zhu S, Zhan J (2013) Combination of solid phase extraction and surface-enhanced Raman spectroscopy for rapid analysis. Analyst 138(9):2598–2603
Y-e Shi, Li L, Yang M, Jiang X, Zhao Q, Zhan J (2014) A disordered silver nanowires membrane for extraction and surface-enhanced Raman spectroscopy detection. Analyst 139(10):2525–2530
Markina NE, Markin AV, Zakharevich AM, Goryacheva IY (2017) Calcium carbonate microparticles with embedded silver and magnetite nanoparticles as new SERS-active sorbent for solid phase extraction. Microchim Acta 184(10):3937–3944
Markina NE, Markin AV, Zakharevich AM, Gorin DA, Rusanova TY, Goryacheva IY (2016) Multifunctional silver nanoparticle-doped silica for solid-phase extraction and surface-enhanced Raman scattering detection. J Nanopart Res 18(12):353
Lee PC, Meisel D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86(17):3391–3395
Leopold N, Lendl B (2003) A new method for fast preparation of highly surface-enhanced Raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J Phys Chem B 107(24):5723–5727
Zhang C, Jiang SZ, Huo YY, Liu AH, Xu SC, Liu XY, Sun ZC, Xu YY, Man BY (2015) SERS detection of R6G based on a novel graphene oxide/silver nanoparticles/silicon pyramid arrays structure. Opt Expresss 23(19):24811–24821
Zhao XM, Zhang BH, Ai KL, Zhang G, Cao LY, Liu XJ, Sun HM, Wang HS, Lu LH (2009) Monitoring catalytic degradation of dye molecules on silver-coated ZnO nanowire arrays by surface-enhanced Raman spectroscopy. J Mater Chem 19(31):5547–5553
SungpyoKim AgaD (2007) Potential ecological and human health impacts of antibiotics and antibiotic-resistant bacteria from wastewater treatment plants. J Toxicol Environ Health B 10(8):559–573
Acknowledgements
This research was supported by the National Natural Science Foundations of China (21505057, 61575087 and 21605062), the Natural Science Foundation of Jiangsu Province (BK20150227, BK20151164), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Brand Major of Universities in Jiangsu Province, and the Top-notch Academic Programs Project of Jiangsu Higher Education Institution (TAPP).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jia, Q., Geng, ZQ., Liu, Y. et al. Highly reproducible solid-phase extraction membrane for removal and surface-enhanced Raman scattering detection of antibiotics. J Mater Sci 53, 14989–14997 (2018). https://doi.org/10.1007/s10853-018-2745-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2745-y