Abstract
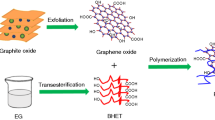
Noncovalent functionalization was used to modify the graphene nanosheets via π–π stacking of pyrene-terminated molecule (Py-LC), resulting in an intercalated layer structure incorporated with about 45 wt% of Py-LC. The organically modified graphene (Py-LC-rGO) has a better dispersion in the PET matrix compared with that without modification. Consequently, it remarkably accelerates the crystallization of PET. Moreover, the nucleation ability of Py-LC-rGO is even stronger than that of commercial nucleation agent, sodium benzoate, leading to a thicker lamellar crystals and denser stacking of lamellae. Accordingly, the impact toughness of such a composite is improved to be nine times as that of neat PET, which can be a promising candidate as nanofiller for PET.













Similar content being viewed by others
References
Lu XF, Hay JN (2001) Isothermal crystallization kinetics and melting behaviour of poly(ethylene terephthalate). Polymer 42:9423–9431
Burgess SK, Leisen JE, Kraftschik BE, Mubarak CR, Kriegel RM, Koros WJ (2014) Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 47:1383–1391
Averett RD, Realff ML, Jacob KI (2009) The effects of fatigue and residual strain on the mechanical behavior of poly(ethylene terephthalate) unreinforced and nanocomposite fibers. Compos A Appl Sci Manuf 40:709–723
Averett RD, Realff ML, Jacob KI (2010) Comparative post fatigue residual property predictions of reinforced and unreinforced poly(ethylene terephthalate) fibers using artificial neural networks. Compos A Appl Sci Manuf 41:331–344
Zhang J, Wu L, Zhao M, Li J, Wang C (2005) Effects of nucleating agents on physical properties of poly(trimethylene terephthalate)/glass-fiber composites. J Appl Polym Sci 96:883–893
Su JJ, Peng F, Gao X, Yang GH, Fu Q, Wang K (2014) Superior toughness obtained via tuning the compatibility of poly(ethylene terephthalate)/poly(ethylene-octene) blends. Mater Des 53:673–680
Xie L, Xie Y, Wu Q, Wang M, Wu Q, Zhou X, Ge X (2015) Effect of poly(acrylic acid)-modified poly(ethylene terephthalate) on improving the integrated mechanical properties of poly(ethylene terephthalate)/elastomer blend. Ind Eng Chem Res 54:4748–4755
Jiang Z, Jin J, Xiao C, Li X (2011) Effect of high content of carbon black on non-isothermal crystallization behavior of poly(ethylene terephthalate). Polym Bull 67:1633–1648
Xia T, Xi Z, Yi X, Liu T, Zhao L (2015) Melt foamability of poly(ethylene terephthalate)/clay nanocomposites prepared by extrusion blending in the presence of pyromellitic dianhydride. Ind Eng Chem Res 54:6922–6931
Shen Z, Luo F, Xing Q, Si P, Lei X, Ji L, Ding S, Wang K (2016) Effect of an aryl amide derivative on the crystallization behaviour and impact toughness of poly(ethylene terephthalate). CrystEngComm 18:2135–2143
Xanthos M, Baltzis BC, Hsu PP (1997) Effects of carbonate salts on crystallization kinetics and properties of recycled poly(ethylene terephthalate). J Appl Polym Sci 64:1423–1435
Todorov LV, Martins CI, Viana JC (2014) In situ waxs/saxs structural evolution study during uniaxial stretching of poly(ethylene terephthalate) nanocomposites in the solid state: Poly(ethylene terephthalate)/titanium dioxide and poly(ethylene terephthalate)/silica nanocomposites. J Appl Polym Sci 131:39752
Xu Y, Song Y, Zheng Q (2015) Effects of nanosilica on crystallization and thermal ageing behaviors of polyethylene terephthalate. Chin J Polym Sci 33:697–708
Wang Y, Wang W, Zhang Z, Xu L, Li P (2016) Study of the glass transition temperature and the mechanical properties of pet/modified silica nanocomposite by molecular dynamics simulation. Eur Polym J 75:36–45
Yin B, Wang J, Jia H, He J, Zhang X, Xu Z (2016) Enhanced mechanical properties and thermal conductivity of styrene-butadiene rubber reinforced with polyvinylpyrrolidone-modified graphene oxide. J Mater Sci 51:5724–5737. doi:10.1007/s10853-016-9874-y
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282–286
Bayrak O, Ionita M, Demirci E, Silberschmidt VV (2016) Effect of morphological state of graphene on mechanical properties of nanocomposites. J Mater Sci 51:4037–4046. doi:10.1007/s10853-016-9722-0
Wang B, Li Y, Weng G, Jiang Z, Chen P, Wang Z, Gu Q (2014) Reduced graphene oxide enhances the crystallization and orientation of poly(ε-caprolactone). Compos Sci Technol 96:63–70
Aoyama S, Park YT, Ougizawa T, Macosko CW (2014) Melt crystallization of poly(ethylene terephthalate): comparing addition of graphene vs. carbon nanotubes. Polymer 55:2077–2085
Xu JZ, Zhang ZJ, Xu H, Chen JB, Ran R, Li ZM (2015) Highly enhanced crystallization kinetics of poly(l-lactic acid) by poly(ethylene glycol) grafted graphene oxide simultaneously as heterogeneous nucleation agent and chain mobility promoter. Macromolecules 48:4891–4900
Mondal T, Ashkar R, Butler P, Bhowmick AK, Krishnamoorti R (2016) Graphene nanocomposites with high molecular weight poly(epsilon-caprolactone) grafts: controlled synthesis and accelerated crystallization. ACS Macro Lett 5:278–282
Seyyed Monfared Zanjani J, Saner Okan B, Menceloglu Y (2016) Manufacturing of multilayer graphene oxide/poly(ethylene terephthalate) nanocomposites with tunable crystallinity, chain orientations and thermal transitions. Mater Chem Phys 176:58–67
Ma WS, Li J, Zhao XS (2013) Improving the thermal and mechanical properties of silicone polymer by incorporating functionalized graphene oxide. J Mater Sci 48:5287–5294. doi:10.1007/s10853-013-7320-y
Criado A, Melchionna M, Marchesan S, Prato M (2015) The covalent functionalization of graphene on substrates. Angew Chem Int Ed 54:10734–10750
Sheng X, Xie D, Cai W, Zhang X, Zhong L, Zhang H (2015) In situ thermal reduction of graphene nanosheets based poly(methyl methacrylate) nanocomposites with effective reinforcements. Ind Eng Chem Res 54:649–658
Wan YJ, Yang WH, Yu SH, Sun R, Wong CP, Liao WH (2016) Covalent polymer functionalization of graphene for improved dielectric properties and thermal stability of epoxy composites. Compos Sci Technol 122:27–35
Santos RM, Vilaverde C, Cunha E, Paiva MC, Covas JA (2016) Probing dispersion and re-agglomeration phenomena upon melt-mixing of polymer-functionalized graphite nanoplates. Soft Matter 12:77–86
Kim MJ, Kim DW, Yun JS, Lee DH, Oh YJ, Nam JA, Kim SR, Lee JH, Park SY, Min BG, In I (2013) Preparation of stable dispersions of chemically reduced graphene oxide through noncovalent interactions with poly(n-isopropyl acrylamide)-grafted pluronic copolymer. J Mater Sci 48:3357–3362. doi:10.1007/s10853-012-7113-8
Ma WS, Wu L, Yang F, Wang SF (2014) Non-covalently modified reduced graphene oxide/polyurethane nanocomposites with good mechanical and thermal properties. J Mater Sci 49:562–571. doi:10.1007/s10853-013-7736-4
Ji L, Wu Y, Ma L, Yang X (2015) Noncovalent functionalization of graphene with pyrene-terminated liquid crystalline polymer. Compos A Appl Sci Manuf 72:32–39
Rohini R, Katti P, Bose S (2015) Tailoring the interface in graphene/thermoset polymer composites: a critical review. Polymer 70:A17–A34
Wang Y, Yang C, Mai YW, Zhang Y (2016) Effect of non-covalent functionalisation on thermal and mechanical properties of graphene-polymer nanocomposites. Carbon 102:311–318
Vasileiou AA, Kontopoulou M, Docoslis A (2014) A noncovalent compatibilization approach to improve the filler dispersion and properties of polyethylene/graphene composites. ACS Appl Mater Interfaces 6:1916–1925
Wan S, Li H, Li D, Xu T, Zhang S, Dou X, Wu L (2015) Noncovalent functionalization of graphene nanosheets with cluster-cored star polymers and their reinforced polymer coating. ACS Macro Lett 4:974–978
Georgakilas V, Tiwari JN, Kemp KC, Perrnan JA, Bourlinos AB, Kim KS, Zboril R (2016) Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev 116:5464–5519
Yu G, Ye Y, Tong Z, Yang J, Li Z, Hua B, Shao L, Li S (2016) A porphyrin-based discrete tetragonal prismatic cage: host-guest complexation and its application in tuning liquid-crystalline behaviour. Macromol Rapid Commun 37:1540–1547
Chen H, Müller MB, Gilmore KJ, Wallace GG, Li D (2008) Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv Mater 20:3557–3561
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217–224
Park S, Lee K-S, Bozoklu G, Cai W, Nguyen ST, Ruoff RS (2008) Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. ACS Nano 2:572–578
Hontorialucas C, Lopezpeinado AJ, Lopezgonzalez JDD, Rojascervantes ML, Martinaranda RM (1995) Study of oxygen-containing groups in a series of graphite oxides: physical and chemical characterization. Carbon 33:1585–1592
Tong ZZ, Zhou B, Huang J, Xu JT, Fan ZQ (2013) Olefinic blocky copolymer/montmorillonite nanocomposites with collapsed clay layers. Compos Sci Technol 85:111–117
Tong ZZ, Zhou B, Huang J, Xu JT, Fan ZQ (2014) Hierarchical structures of olefinic blocky copolymer/montmorillonite nanocomposites with collapsed and intercalated clay layers. RSC Adv 4:15678–15688
Tong ZZ, Huang J, Zhou B, Xu JT, Fan ZQ (2015) Self-nucleation behaviors of olefinic blocky copolymer/montmorillonite nanocomposites with collapsed and intercalated clay layers. J Appl Polym Sci 132:41771
Jiang XL, Luo SJ, Sun K, Chen XD (2007) Effect of nucleating agents on crystallization kinetics of PET. Expr Polym Lett 1:245–251
Avrami M (1939) Kinetics of phase change I: general theory. J Chem Phys 7:1103–1112
Huang HD, Xu JZ, Fan Y, Xu L, Li ZM (2013) Poly(L-lactic acid) crystallization in a confined space containing graphene oxide nanosheets. J Phys Chem B 117:10641–10651
Strobl GR, Schneider MJ, Voigt-Martin IG (1980) Model of partial crystallization and melting derived from small-angle X-ray scattering and electron microscopic studies on low-density polyethylene. J Polym Sci B Polym Phys 18:1361–1381
Strobl GR, Schneider MJ (1980) Direct evaluation of the electron density correlation function of partially crystalline polymers. J Polym Sci B Polym Phys 18:1343–1359
Tong ZZ, Xu JT, Xia SJ, Fan ZQ (2013) Comparison of chain structure and morphology of an olefinic blocky copolymer and a Ziegler–Natta-based ethylene random copolymer. Polym Int 62:228–237
Tong ZZ, Huang J, Zhou B, Xu JT, Fan ZQ (2013) Chain microstructure, crystallization, and morphology of olefinic blocky copolymers. Macromol Chem Phys 214:605–616
Tong ZZ, Zhou B, Huang J, Xu JT, Fan ZQ (2014) Regulation of crystallization kinetics, morphology, and mechanical properties of olefinic blocky copolymers. Macromolecules 47:333–346
Liu YM, Tong ZZ, Xu JT, Fu ZS, Fan ZQ (2014) A highly efficient beta-nucleating agent for impact-resistant polypropylene copolymer. J Appl Polym Sci 131:40753
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21604073), Public Welfare Technology Application Research Project of Zhejiang Province (2017C31026), the Science Foundation of Zhejiang Sci-Tech University (ZSTU) under Grant No. 15012082-Y, Zhejiang Top Priority Discipline of Textile Science and Engineering (2014YBZX02), the Young Researchers Foundation of Key Laboratory of Advanced Textile Materials and Manufacturing Technology, Ministry of Education, Zhejiang Sci-Tech University (2016QN01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material including DSC curves, UV–Vis spectra, synchrotron WAXS profile, and photograph of dispersion of graphene.
Rights and permissions
About this article
Cite this article
Tong, Z., Zhuo, W., Zhou, J. et al. Crystallization behavior and enhanced toughness of poly(ethylene terephthalate) composite with noncovalent modified graphene functionalized by pyrene-terminated molecules: a comparative study. J Mater Sci 52, 10567–10580 (2017). https://doi.org/10.1007/s10853-017-1173-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1173-8