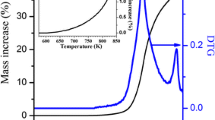
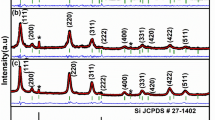
Abstract
Metal-doped magnesium silicides are promising thermoelectric materials for waste heat recovery application at 500–800 K because of their low density, large natural availability, non-toxicity, good thermal stability, and transport properties. Reaction kinetics of metal-doped magnesium silicides, Mg2SiX m (X = Ti, Nb, Mn, and Co; m = 0.02, 0.04, and 0.08 mol) were investigated in this study. A simple and rapid synthesis of Mg2SiX m samples was carried out using pelletizing, and sintering method at 773–823 K for 300 s. The effect of metal doping on the lattice constants of Mg2SiX m samples was examined using X-ray diffraction technique. Differential thermal analysis heat flow experiments were conducted on (2Mg + Si + mX) sample mixtures to study the solid-state reaction kinetics of Mg2SiX m alloys formation at different scan rates of 0.08, 0.16, 0.25, 0.33 Ks−1. Activation energies for the formation reaction of Mg2Si were determined using Ozawa, and Kissinger–Akahira–Sunrose equations. A 3-D diffusion-controlled reaction mechanism was proposed based on Coats–Redfern (CR) model. The effect of concentration of the metal-dopants on the formation activation energies of Mg2SiX m was investigated using the CR equation plots.










Similar content being viewed by others
References
Seebeck TJ (1826) Ueber die magnetische Polarisation der metalle und erze durch temperaturdifferenz. Ann Phys 82:253–286
Peltier JCA (1834) Nouvelles expériences sur la caloricité des courants électirique. Ann Chim Phys 56:371–386
Rosi FD (1968) Thermoelectricity and thermoelectric power generation. Solid State Electron 11:833–868
Tritt TM, Böttner H, Chen L (2008) Thermoelectrics: direct solar thermal energy conversion. MRS Bull 33:366–368
Minnich A, Dresselhaus MS, Ren ZF et al (2009) Bulk nanostructured thermoelectric materials: current research and future prospects. Energy Environ Sci 2:466–479
Snyder GJ, Toberer ES (2008) Complex thermoelectric materials. Nat Mater 7:105–114
Rowe DM (2005) Thermoelectrics handbook: macro to nano, 1st edn. CRC Taylor & Francis, Boca Raton
Lange H (1997) Electronic properties of semiconducting silicides. Phys Status Solidi B 201:3–65
Ivanenko LI, Shaposhnikov VL, Filonov AB et al (2004) Electronic properties of semiconducting silicides: fundamentals and recent predictions. Thin Solid Films 461:141–147
Nikitin EN (1958) Study of temperature dependencies of electrical conductivity and thermal power of silicides. Zhur Tekhn Fiz 28:23
Fedorov MI, Zaitsev VK (2008) The features of silicide thermoelectrics development. In: 6th European conference on thermoelectrics (ECT2008), I-11:1–6
Fedorov MI (2009) Thermoelectric silicides: past, present and future. J Thermoelectr 2:51–60
Fedorov MI, Isachenko GN (2015) Silicides: materials for thermoelectric energy conversion. Jpn J Appl Phys 54(07JA05):1–6
Bogala MR, Reddy RG (2015) Phase stability of thermoelectric alkaline earth metal borides and silicides. In: The Minerals, Metals & Materials Society (eds) TMS 2015 144th Annual Meeting & Exhibition. Springer, Cham
Bogala MR, Reddy RG (2016) Synthesis, characterization and Gibbs energy of thermoelectric Mg2Si. Ceram Trans 259:143–152
Bux SK, Yeung MT, Toberer ES et al (2011) Mechanochemical synthesis and thermoelectric properties of high quality magnesium silicide. J Mater Chem 21:12259–12266
Villars P (2014) Springer & Material Phases Data System (MPDS). http://materials.springer.com/isp/crystallographic/docs/sd_0531570. Accessed 22 Oct 2014
Wang L, Qin XY (2003) The effect of mechanical milling on the formation of nanocrystalline Mg2Si through solid-state reaction. Scr Mater 49:243–248
Bale CW, Bélisle E, Chartrand P et al (2009) FactSage thermochemical software and databases—recent developments. http://www.factsage.com/. Accessed 16 May 2015
Jain A, Ong SP, Hautier G et al (2013) The materials project: a materials genome approach to accelerating materials innovation. https://materialsproject.org/materials/mp-1367/. Accessed 28 June 2015
Lee HJ, Cho YR, Kim I (2011) Synthesis of thermoelectric Mg2Si by a solid state reaction. J Ceram Process Res 12:16–20
Tani J, Kido H (2005) Thermoelectric properties of Bi-doped Mg2Si semiconductors. Phys B Condens Matter 364:218–224
You S, Park K, Kim I et al (2012) Solid-state synthesis and thermoelectric properties of Al-doped Mg2Si. J Electron Mater 41:1675–1679
Tani J, Kido H (2007) Thermoelectric properties of Sb-doped Mg2Si semiconductors. Intermetallics 15:1202–1207
Fan W, Chen R, Wang L et al (2011) First-principles and experimental studies of Y-doped Mg2Si prepared using field-activated pressure-assisted synthesis. J Electron Mater 40:1209–1214
Akasaka M, Iida T, Matsumoto A et al (2008) The thermoelectric properties of bulk crystalline n-and p-type Mg2Si prepared by the vertical Bridgman method. J Appl Phys 104:013703
Bercegol A, Christophe V, Keshavarz MK et al (2016) Hot extruded polycrystalline Mg2Si with embedded XS2 nano-particles (X: Mo, W). J Electron Mater. doi:10.1007/s11664-016-4868-8
Satyala N, Krasinski JS, Vashaee D (2014) Simultaneous enhancement of mechanical and thermoelectric properties of polycrystalline magnesium silicide with conductive glass inclusion. Acta Mater 74:141–150
Fiameni S, Battiston S, Boldrini S et al (2012) Synthesis and characterization of Bi-doped Mg2Si thermoelectric materials. J Solid State Chem 193:142–146
You S, Kim I (2011) Solid-state synthesis and thermoelectric properties of Bi-doped Mg2Si compounds. Curr Appl Phys 11:S392–S395
Battiston S, Fiameni S, Saleemi M et al (2013) Synthesis and characterization of Al-doped Mg2Si thermoelectric materials. J Electron Mater 42:1956–1959
Ioannou M, Polymeris G, Hatzikraniotis E et al (2013) Solid-state synthesis and thermoelectric properties of Sb-doped Mg2Si materials. J Electron Mater 42:1827–1834
Zaitsev VK, Fedorov MI, Gurieva EA et al (2006) Highly effective Mg2Si1−x Sn x thermoelectrics. Phys Rev B 74(045207):1–5
Jiang H, Long H, Zhang L (2004) Effects of solid-state reaction synthesis processing parameters on thermoelectric properties of Mg2Si. J Wuhan Univ Technol Mater Sci Ed 19:55–56
Khajelakzay M, Bakhshi SR, Borhani GH et al (2016) Synthesis and spark plasma sintering of Mg2Si nanopowder by mechanical alloying and heat treatment. Int J Appl Ceram Technol 13:498–505
Ito M, Kawahara K (2015) Synthesis of thermoelectric Mg2Si by reactive sintering utilizing directly applied current sintering. Mater Trans 56:2023–2028
Meng QS, Fan WH, Chen RX et al (2011) Thermoelectric properties of Sc-and Y-doped Mg2Si prepared by field-activated and pressure-assisted reactive sintering. J Alloys Compd 509:7922–7926
Yoshinaga M, Iida T, Noda M et al (2004) Bulk crystal growth of Mg2Si by the vertical Bridgman method. Thin Solid Films 461:86–89
Fu G, Zuo L, Longtin J et al (2013) Thermoelectric properties of magnesium silicide fabricated using vacuum plasma thermal spray. J Appl Phys 114(144905):1–6
Berthebaud D, Gascoin F (2013) Microwaved assisted fast synthesis of n and p-doped Mg2Si. J Solid State Chem 202:61–64
Niu X, Lu L (1997) Formation of magnesium silicide by mechanical alloying. Adv Perform Mater 4:275–283
Horvitz D, Klinger L, Gotman I (2004) New approach to measuring the activation energy of thermal explosion and its application to Mg–Si system. Scr Mater 50:631–634
Sun B, Li S, Imai H et al (2012) Synthesis kinetics of Mg2Si and solid-state formation of Mg–Mg2Si composite. Powder Technol 217:157–162
Ozawa T (1970) Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim 2:301–324
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Ioannou M, Chrissafis K, Pavlidou E et al (2013) Solid-state synthesis of Mg2Si via short-duration ball-milling and low-temperature annealing. J Solid State Chem 197:172–180
Acknowledgements
The authors are thankful to the National Science Foundation (NSF) agency for the financial support from the Grant No. DMR-1310072, American Cast Iron Pipe Company (ACIPCO), and Department of Metallurgical and Materials Engineering (MTE) at the University of Alabama for providing the central analytical facilities to complete the present research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest associated with the studies mentioned in the current research article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bogala, M.R., Reddy, R.G. Reaction kinetic studies of metal-doped magnesium silicides. J Mater Sci 52, 11962–11976 (2017). https://doi.org/10.1007/s10853-017-1095-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1095-5