Abstract
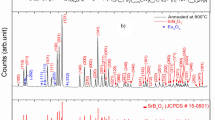
Crystalline Tb2(CO3)3: Eu3+ samples were successfully synthesized by the precipitation reaction of rare-earth chloride with ammonium bicarbonate in solution directly under mild condition without further thermal treatment. The samples were characterized by means of X-ray diffraction, scanning electron microscopy, transmission electron microscopy, thermogravimetry analysis, Fourier transform infrared spectroscopy, photoluminescence, as well as lifetimes. Influences of pH, molar ratio of precipitant to rare earth ions, aging time, temperature, and surfactant on the morphology and crystal structure were investigated in detail. The obtained samples presented dumbbell-like microstructures which were assembled from nanosheets with the assistance of ethylene glycol. Under the excitation of 220-nm ultraviolet light, the Tb2(CO3)3 samples showed the characteristic emissions of Tb3+ corresponding to 5D4 → 7F6,5,4,3 transitions, whereas the Tb2(CO3)3: Eu3+ samples mainly exhibited the characteristic emissions of Eu3+ corresponding to 5D0 → 7F0,1,2,3,4 transitions due to an effective energy transfer from Tb3+ to Eu3+. The energy transfer efficiency from Tb3+ to Eu3+ increased with Eu3+ doping concentration. The multicolor emission of Tb2(CO3)3: Eu3+ samples can be tuned from green to red easily by altering the doping concentration of Eu3+. The materials are expected to apply widely in the future, and the simple method is particularly suitable for large-scale industrial production.

















Similar content being viewed by others
References
Yu M, Lin J, Fu J, Zhang HJ (2003) Sol-gel synthesis and photoluminescent properties of LaPO4: A (A = Eu3+, Ce3+, Tb3+) nanocrystalline thin films. J Mater Chem 13:1413–1419
Capobianco JA, Vetrone F, Boyer JC, Speghini A, Bettinelli M (2002) Visible upconversion of Er3+ doped nanocrystalline and bulk Lu2O3. Opt Mater 19:259–268
Feng W, Sun LD, Zhang YW, Yan CH (2010) Synthesis and assembly of rare earth nanostructures directed by the principle of coordination chemistry in solution based process. Coord Chem Rev 254:1038–1053
Wang G, Peng Q, Li Y (2011) Lanthanide-doped nanocrystals: synthesis, optical-magnetic properties, and applications. Acc Chem Res 44:322–332
Li CX, Lin J (2010) Rare earth fluoride nano-/microcrystals: synthesis, surface modification and application. J Mater Chem 20:6831–6847
Bouzigues C, Gacoin T, Alexandrou A (2011) Biological applications of rare earth based nanoparticles. ACS Nano 5:8488–8505
Baldo MA, O’Brien DF, You Y, Shoustikov A et al (1998) Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395:151–154
Dabbousi BO, RodriguezViejo J, Mikulec FV et al (1997) (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B 46:9463–9475
Wang GF, Peng Q, Li YD (2009) Upconversion Luminescence of Monodisperse CaF2: Yb3+/Er3+ Nanocrystals. J Am Chem Soc 131:14200–14201
Li P, Peng Q, Li YD (2009) Dual-mode luminescent colloidal spheres from monodisperse rare-earth fluoride nanocrystals. Adv Mater 21:1945–1948
Wang F, Liu XG (2009) Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem Soc Rev 38:976–989
Sun LN, Mai WP, Dang S, Qiu YN, Deng W, Shi LY, Yan W, Zhang HJ (2012) Near-infrared luminescence of periodic mesoporous organosilicas grafted with lanthanide complexes based on visible-light sensitization. J Mater Chem 22:5121–5127
Spassky D, Ivanov S, Kitaeva I, Kolobanov V, Mikhailin V, Ivleva L, Voronina I (2005) Optical and luminescent properties of a series of molybdate single crystals of scheelite crystal structure. Phys Status Solidi C 2:65–68
Salutsky ML, Quill LL (1950) The rare earth metals and their compounds. XII. Carbonates of lanthanum, neodymium and samarium. J Am Chem Soc 72:3306–3307
Sastry RLN, Yoganarasimhan SR (1966) Preparation, characterization and thermal decomposition of praseodymium, terbium and neodymium carbonates. J Inorg Nucl Chem 28:1165–1177
Wakita H, Nagashima K (1972) Synthesis of tengerite type rare earth carbonates. Bull Chem Soc Jpn 45:2476–2479
Mochizuki A, Nagashima K, Wakita H (1974) The synthesis of crystalline hyclrated double carbonates of rare earth elements and sodium. B Chem Soc Jpn 47:755–756
Nagashima K, Wakita H, Mochizuki A (1973) The synthesis of crystalline rare earth carbonates. Bull Chem Soc Jpn 46:152–156
Charles RG (1965) Rare-earth carbonates prepared by homogeneous precipitation. J Inorg Nucl Chem 27:1489–1493
Moeller T, Horwitz EP (1959) Some characteristics of ethylenediaminetetraacetic acid, N-hydroxyethylethylenediaminetriacetic acid, and 1,2-diaminocyclohexanetetraacetic acid chelates of certain rare-earth metal ions. J Inorg Nucl Chem 12:49–59
Head EL, Holley CE (1964) The thermal decomposition of scandium formate and oxalate. J Inorg Nucl Chem 26:525–530
Head EL (1966) preparation of the carbonates of the rare earths from some of their organic acid salts. Inorg Nucl Chem Lett 2:33–37
Tareen JAK, Viswanathiah MN, Krishnamurthy KV (1980) Hydrothermal synthesis and growth of Y(OH)CO3-ancylite like phase. Rev Chim Miner 17:50–57
Tareen JAK, Kutty TRN (1980) Hydrothermal phase equilibria in Ln2O3-H2O-CO2 systems: I. The lighter lanthanides. J Cryst Growth 50:527–532
Tareen JAK, Narayanan TR, Kutty TRN (1980) Hydrothermal growth of Y2(CO3)3·nH2O (tengerite) single crystals. J Cryst Growth 49:761–765
Tareen JAK, Basavalingu B, Kutty TRN (1981) Hydrothermal synthesis of polycrystalline carbonates. J Cryst Growth 55:384–387
Wakita H, Kinoshita S (1979) Synthetic study of the solid-solutions in the systems La2(CO3)3·8H2O–Ce2(CO3)3·8H2O and La(OH)CO3–Ce(OH)CO3. Bull Chem Soc Jpn 52:428–432
Wakita H (1978) The synthesis of hydrated rare earth carbonate single crystals in gels. Bull Chem Soc Jpn 51:2879–2881
Liu S, Ma RJ (1999) Precipitation and characterization of cerous carbonate. J Cryst Growth 206:88–92
Liu S, Ma RJ (1996) Synthesis and structure of hydrated europium carbonate. J Cryst Growth 169:190–192
Palmer MS, Neurock M, Olken MM (2002) Periodic density functional theory study of methane activation over La2O3: activity of O2-, O-, O22-, oxygen point defect, and Sr2+ -doped surface sites. J Am Chem Soc 124:8452–8461
Chen W, Sammynaiken R, Huang YN (2000) Photoluminescence and photostimulated luminescence of Tb3+ and Eu3+ in zeolite-Y. J Appl Phys 88:1424–1431
Li GG, Hou ZY, Peng C, Wang WX, Cheng ZY, Li CX, Lian HZ, Lin J (2000) Electrospinning derived one-dimensional LaOCl: Ln3+ (Ln = Eu/Sm, Tb, Tm) nanofibers, nanotubes and microbelts with multicolor-tunable emission properties. Adv Funct Mater 20:3446–3456
Zhong JM, Zhao WR, Song EH, Deng YQ (2014) Luminescence properties and dynamical processes of energy transfer in BiPO4: Tb3+, Eu3+ phosphor. J Lumin 154:204–210
Di WH, Wang XJ, Zhu PF, Chen BJ (2007) Energy transfer and heat-treatment effect of photoluminescence in Eu3+-doped TbPO4 nanowires. J Solid State Chem 180:467–473
Kim Anh T, Strek W (1988) Dynamics of energy transfer in Tb1−x Eu x P5O14 crystals. J Lumin 42:205–210
Schierning G, Batentschuk M, Osvet A, Winnacker A (2004) On the energy transfer from Tb3+ to Eu3+ in LiTb1−x Eu x P4O12. Radiat Meas 38:529–532
Yang J, Zhang CM, Li CX, Yu YN, Lin J (2008) Energy transfer and tunable luminescence properties of Eu3+ in TbBO3 microspheres via a facile hydrothermal process. Inorg Chem 47:7262–7270
Hou ZY, Cheng ZY, Li GG, Lin J (2011) Electrospinning-derived Tb2(WO4)3: Eu3+ nanowires: energy transfer and tunable luminescence properties. Nanoscale 3:1568–1574
Yue D, Lu W, Jin L, Li CY et al (2014) Controlled synthesis, asymmetrical transport behavior and luminescence properties of lanthanide doped ZnO mushroom-like 3D hierarchical structures. Nanoscale 6:13795–13802
Gai SL, Li CX, Yang PP, Lin J (2014) Recent progress in rare earth micro/nanocrystals: soft chemical synthesis, luminescent properties, and biomedical applications. Chem Rev 114:2343–2389
Zhang Y, Hao JH (2013) Metal-ion doped luminescent thin films for optoelectronic applications. J Mater Chem C 1:5607–5618
Tsang MK, Bai GX, Hao JH (2015) Stimuli responsive upconversion luminescence nanomaterials and films for various applications. Chem Soc Rev. doi:10.1039/C4CS00171K
Bai GX, Tsang MK, Hao JH (2014) Tuning the luminescence of phosphors: beyond conventional chemical method. Adv Opt Mater. doi:10.1002/adom.201400375
Liu S, Ma RJ (1996) Synthesis and structure of hydrated terbium carbonate. Indian J Chem 35:992–994
Sungur A, Kizilyalli M (1983) Synthesis and structure of Gd2(CO3)3·2H2O, Gd2(CO3)3·3H2O. J Less-Common Met 93:419–423
Li YP, Zhang JH, Zhang X, Luo YS, Lu SZ, Ren XG, Wang XJ, Sun LD, Yan CH (2009) Luminescent properties in relation to controllable phase and morphology of LuBO3: Eu3+ nano/microcrystals synthesized by hydrothermal approach. Chem Mater 21:468–475
Tian Y, Chen BJ, Tian BN, Yu NS (2013) Hydrothermal synthesis and tunable luminescence of persimmon-like sodium lanthanum tungstate: Tb3+, Eu3+ hierarchical microarchitectures. J Colloid Interf Sci 393:44–52
Li CX, Zhang CM, Hou ZY, Wang LL, Quan ZW, Lian HZ, Lin J (2009) β-NaYF4 and β-NaYF4: Eu3+ microstructures: morphology control and tunable luminescence properties. J Phys Chem C 113:2332–2339
Li CX, Yang J, Quan ZW, Yang PP, Kong DY, Lin J (2007) Different microstructures of β-NaYF4 fabricated by hydrothermal process: effects of ph values and fluoride sources. Chem Mater 19:4933–4942
Yu CZ, Fan J, Tian BZ, Zhao DY (2004) Morphology development of mesoporous materials: a colloidal phase separation mechanism. Chem Mater 16:889–898
Lee S, Song D, Kim D, Lee J, Kim S, Park IY, Choi YD (2004) Effects of synthesis temperature on particle size/shape and photoluminescence characteristics of ZnS: Cu nanocrystals. Mater Lett 58:342–346
Galea L, Bohner M, Thuering J, Doebelin N, Aneziris CG, Graule T (2013) Control of the size, shape and composition of highly uniform, non-agglomerated, sub-micrometer β-tricalcium phosphate and dicalcium phosphate platelets. Biomaterials 34:6388–6401
Tseng TK, Choi JH, Jung DW, Davidson M, Holloway PH (2010) Three-dimensional self-assembled hierarchical architectures of gamma-phase flowerlike bism three-dimensional self-assembled hierarchical architectures of gamma-phase flowerlike bismuth oxideuth oxide. ACS Appl Mater Interfaces 2:943–94650
Jia ZG, Yang LL, Wang QZ, Liu JH (2014) Synthesis of hierarchical CoFe2O4 nanorod-assembled superstructures and its catalytic application. Mater Chem Phys 145:116–124
Lim BK, Xiong YJ, Xia YN (2007) A water-based synthesis of octahedral, decahedral, and icosahedral Pd nanocrystals. Angew Chem Int Ed 119:9279–9282
Dexter DL (1953) A theory of sensitized luminescence in solids. J Chem Phys 21:836–850
Huang CH, Chen TM (2010) Ca9La(PO4)7: Eu2+, Mn2+: an emission-tunable phosphor through efficient energy transfer for white light-emitting diodes. Opt Express 18:5089–5099
Wang YL, Jiang XC, Xia YN (2003) A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J Am Chem Soc 125:16176–16177
Jiang LH, Sun GQ, Zhou ZH, Sun SG et al (2005) Size-controllable synthesis of monodispersed SnO2 nanoparticles and application in electrocatalysts. J Phys Chem B 109:8774–8778
Burda C, Chen XB, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102
Zhu LP, Zhang WD, Xiao HM, Yang Y, Fu SY (2008) Facile synthesis of metallic co hierarchical nanostructured microspheres by a simple solvothermal process. J Phys Chem C 112:10073–10078
Huang SH, Zhang X, Wang LZ, Bai L (2012) Controllable synthesis and tunable luminescence properties of Y2(WO4)3: Ln3+ (Ln = Eu, Yb/Er, Yb/Tm and Yb/Ho) 3D hierarchical architectures. Dalton Trans 41:5634–5642
Politi Y, Arad T, Klein E, Weiner S, Addadi L (2004) Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science 306:1161–1164
Cölfen H, Antonietti M (2005) Mesocrystals: inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew Chem Int Ed 44:5576–5591
Zhang N, Bu WB, Xu YP, Jiang DY, Shi JL (2007) Self-assembled flowerlike europium-doped lanthanide molybdate microarchitectures and their photoluminescence properties. J Phys Chem C 111:5014–5019
Wang YH, Huang Y (2014) Enhanced green emission of Eu2+ by energy transfer from the 5D3 Level of Tb3+ in NaCaPO4. J Phys Chem C 118:7002–7009
Thomas KS, Singh S, Dieke GH (1963) Energy levels of Tb3+ in LaCl3 and other chlorides. J Chem Phys 38:2180–2190
Nakazawa E, Shionoya S (1967) Energy transfer between trivalent rare-earth ions in inorganic solids. J Chem Phys 47:3211–3219
Hou L, Cui SB, Fu ZL et al (2014) Facile template free synthesis of KLa(MoO4)2: Eu3+, Tb3+ microspheres and their multicolor tunable luminescence. Dalton Trans 43:5382–5392
Kumar GA, Biju PR, Jose G, Unnikrishnan NV (1999) Static energy transfer for Mn2+: Pr3+ system in phosphate glasses. Mater Chem Phys 60:247–255
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (51302229 and 21204074), the Natural Science Foundation Project of CQ CSTC (cstc2012jjA50009), the Fundamental Research Funds for the Central Universities (XDJK2013B016), the China Postdoctoral Science Foundation funded project (2012M521663), the Research Fund for the Doctoral Program of Higher Education of China (20120182120018), the Scientific Research Foundation for Returned Scholars, Ministry of Education of China (46th), the Postdoctoral Scientific Research Project Special Funding of Chongqing (Xm201312), and the Foundation of State Key Laboratory of Rare Earth Resources Utilization (RERU2013015).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, L., Yang, J., Hu, S. et al. Synthesis of 3D hierarchical architectures of Tb2(CO3)3: Eu3+ phosphor and its efficient energy transfer from Tb3+ to Eu3+ . J Mater Sci 50, 4503–4515 (2015). https://doi.org/10.1007/s10853-015-9000-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9000-6