Abstract
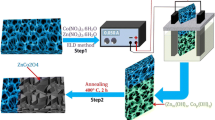
Aluminum-substituted α-Ni(OH)2 nanoflakes grown on nickel foam as electrode for application in supercapacitor were synthesized in the presence of urea through a hydrothermal route. The as-synthesized nanoflakes with ultra-thin thickness of about 12 nm presented isolated state on Nickel foam. The sample substituted with 9.8 % Al showed the highest specific capacitance of 3637 F g−1 at scan rate of 5 mV s−1 in 3 M KOH aqueous solution. This sample also kept high specific capacitance of 1142 F g−1 at high charge and discharge current density of 32 A g−1. The excellent electrochemical performance can be ascribed to the thin thickness and isolated state of these nanoflakes. The two characteristics of nanoflakes guaranteed their full contact with electrolyte during the electrochemical reactions, therefore leading to the instant and full utilization of electroactive material. During stability test, the capacitance of material still remained 81 % after 2000 charge–discharge cycles. These results demonstrated that the nanoflakes could be applied as high performance electrode material in supercapacitor.






Similar content being viewed by others
References
Li YM, Li WY, Chou SL, Chen J (2008) Synthesis, characterization and electrochemical properties of aluminum-substituted alpha-Ni(OH)2 hollow spheres. J Alloys Compd 456:339–343
Xu QS, Zhu YJ, Han QY, Zhao RD, Zhuang YH (2014) Preparation of Yb-substituted α-Ni(OH)2 and its physicochemical properties. J Alloys Compd 584:1–6
Kazimirov VY, Smirnov MB, Bourgeois L, Guerlou-Demourgues L, Servant L, Balagurov AM, Natkaniec I, Khasanova NR, Antipov EV (2010) Atomic structure and lattice dynamics of Ni and Mg hydroxides. Solid State Ionics 181:1764–1770
Jiang H, Zhao T, Li CZ, Ma J (2011) Hierarchical self-assembly of ultrathin nickel hydroxide nanoflakes for high-performance supercapacitors. J Mater Chem 21:3818–3823
Yang DN, Wang RM, He MS, Zhang J, Liu ZF (2005) Ribbon and boardlike nanostructures of nickel hydroxide: synthesis, characterization, and electrochemical properties. J Phys Chem B 109:7654–7658
Hu WK, Gao XP, Noréus D, Burchardt T, Nakstad NK (2006) Evaluation of nano-crystal sized & α-nickel hydroxide as an electrode material for alkaline rechargeable cells. J Power Sources 160:704–710
Hu WK, Nore D (2003) Alpha nickel hydroxides as lightweight nickel electrode materials for alkaline rechargeable cells. Chem Mater 15:974–978
Aghazadeh M, Ghaemi M, Sabour B, Dalvand S (2014) Electrochemical preparation of α-Ni(OH)2 ultrafine nanoparticles for high-performance supercapacitors. J Solid State Electrochem 18:1569–1584
Kong XG, Zhao JW, Shi WY, Zhao YF, Shao MF, Wei M, Wang LR, Duan X (2012) Fabrication of aluminum-doped & α-Ni(OH)2 with hierarchical architecture and its largely enhanced electrocatalytic performance. Electrochim Acta 80:257–263
Wang LQ, Li XC, Guo TM, Yan XB, Tayc BK (2014) Three-dimensional Ni(OH)2 nanoflakes/graphene/nickel foam electrode with high rate capability for supercapacitor applications. Int J Hydrogen Energ 39:7876–7884
Yang GW, Xu CL, Li HL (2008) Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem Commun 48:6537–6539
Zhao YL, Wang JM, Chen H, Pan T, Zhang JQ, Cao CN (2004) Different additives-substituted α-nickel hydroxide prepared by urea decomposition. Electrochim Acta 50:91–98
Huang JC, Cao DX, Lei T, Yang SN, Zhou XB, Xu PP, Wang GL (2013) Structural and electrochemical performance of Al-substituted β-Ni(OH)2 nanosheets electrodes for nickel metal hydride battery. Electrochim Acta 111:713–719
Yan L, Li RY, Liu ZJ, Fang YJ, Wang GL, Gu ZG (2013) Three-dimensional activated reduced graphene oxide nanocup/nickel aluminum layered double hydroxides composite with super high electrochemical and capacitance performances. Electrochim Acta 95:146–154
Zhao YL, Wang JM, Chen H, Pan T, Zhang JQ, Cao CN (2004) Al-substituted α-nickel hydroxide prepared by homogeneous precipitation method with urea. Int J Hydrogen Energy 29:889–896
Wang J, Song YC, Li ZS, Liu Q, Zhou JD, Jing XY, Zhang ML, Jiang ZH (2010) In situ Ni/Al layered double hydroxide and its electrochemical capacitance performance. Energy Fuels 24:6463–6467
Chen H, Hu LF, Chen M, Yan YM, Wu L (2014) Nickel–Cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv Funct Mater 24:934–942
Chen H, Hu LF, Yan Y, Che RC, Chen M, Wu LM (2013) One-step fabrication of ultrathin porous nickel hydroxide- manganese dioxide hybrid nanosheets for supercapacitor electrodes with excellent capacitive performance. Adv Energy Mater 3:1636–1646
Chen H, Wang JM, Pan T, Zhao YL, Zhang JQ, Cao CN (2005) The structure and electrochemical performance of spherical Al-substituted α-Ni(OH)2 for alkaline rechargeable batteries. J Power Sources 143:243–255
Yang LJ, Gao XP, Wu QD, Zhu HY, Pan GL (2007) Phase distribution and electrochemical properties of Al-substituted nickel hydroxides. J Phys Chem C 111:4614–4619
Huang JC, Lei T, Wei XP, Liu XW, Liu T, Cao DX, Yin JL, Wang GL (2013) Effect of Al-doped β-Ni(OH)2 nanosheets on electrochemical behaviors for high performance supercapacitor application. J Power Sources 232:370–375
He F, Hu ZB, Liu KY, Zhang SR, Liu HT, Sang SB (2014) In situ fabrication of nickel aluminum-layered double hydroxide nanosheets/hollow carbon nanofibers composite as a novel electrode material for supercapacitors. J Power Sources 267:188–196
Min SD, Zhao CJ, Chen GR, Qian XZ (2014) One-pot hydrothermal synthesis of reduced graphene oxide/Ni(OH)2 films on nickel foam for high performance supercapacitors. Electrochim Acta 115:155–164
Yan J, Fan ZJ, Sun W, Ning GQ, Wei T, Zhang Q, Zhang RF, Zhi LJ, Wei F (2012) Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv Funct Mater 22:2632–2641
Zhi J, Deng S, Zhang YX, Wang YF, Hu AG (2013) Embedding Co3O4 nanoparticles in SBA-15 supported carbon nanomembrane for advanced supercapacitor materials. J Mater Chem A 1:3171–3176
Zhu JX, Shi WH, Xiao N, Rui XH, Tan HT, Lu XH, Ma J, Yan QY (2012) Oxidation-etching preparation of MnO2 tubular nanostructures for high-performance supercapacitors. ACS Appl Mater Inter 4:2769–2774
Chen W, Rakhi RB, Hu LB, Xie X (2011) High performance nanostructured supercapacitors on a sponge. Nano Lett 11:5165–5172
Wu ZS, Wang DW, Ren W, Zhao J, Zhou G, Li F, Cheng HM (2010) Anchoring hydrous RuO2 on graphene sheets for high-performance electrochemical capacitors. Adv Funct Mater 20:3595–3602
Wang H, Casalongue HS, Liang Y, Dai H (2010) Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J Am Chem Soc 132:7472–7477
Wu Z, Huang XL, Wang ZL, Xu JJ, Wang HG, Zhang XB (2014) Electrostatic induced stretch growth of homogeneous β-Ni(OH)2 on graphene with enhanced high-rate cycling for supercapacitors. Sci Rep 4:3669–3676
Niu YL, Li RY, Li ZJ, Fang YJ, Liu JK (2013) High-performance supercapacitors materials prepared via in situ growth of NiAl-layered double hydroxide nanoflakes on well-activated graphene nanosheets. Electrochim Acta 94:360–366
Gao Z, Wang J, Li ZS, Yang WL, Wang B, Hou MJ, He Y, Liu Q, Mann T, Yang PP, Zhang ML, Liu LH (2011) Graphene nanosheet/Ni2+/Al3+ layered double-hydroxide composite as a novel electrode for a supercapacitor. Chem Mater 23:3509–3516
Wang D, Yan W, Botte GG (2011) Exfoliated nickel hydroxide nanosheets for urea electrolysis. Electrochem Commun 13:1135–1138
Fang JH, Li M, Li QQ, Zhang WF, Shou QL, Liu F, Zhang XB, Cheng JP (2012) Microwave-assisted synthesis of CoAl-layered double hydroxide/graphene oxide composite and its application in supercapacitors. Electrochim Acta 85:248–255
Acknowledgement
This work was financially supported by Shandong Provincial Engineering Research Center for Comprehensive Utilization of Light Hydrocarbons.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Xue, J., Zhang, F. et al. Growth of aluminum-substituted nickel hydroxide nanoflakes on nickel foam with ultrahigh specific capacitance at high current density. J Mater Sci 50, 2422–2428 (2015). https://doi.org/10.1007/s10853-014-8797-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8797-8