Abstract
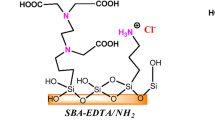
This research proposed the use of a mesoporous silica material (SiO2) as a Cu(I) adsorbent in a pre-treatment of cyanide effluents employed in gold and silver extraction. Two copper sources were employed: a [Cu(CN) X ]−(X+1) standard solution, and a cyanide solution obtained from an ore of Peña de Bernal, Chihuahua, México, which was named Cu(I)–CN–PB. Mesoporous silica removes around 90 % of the Cu(I)–CN at 30 min in Cu(I)–CN solutions with 50 ppm of the metals; while, in a solution with a high concentration of copper (311 ppm), around 52 % was removed. The adsorption dates were adjusted following the Langmuir model; obtained a maximum adsorption capacity (Q 0) of 8.01 mg g−1 and a separation factor (R L) lower than one, which indicates a favorable thermodynamic adsorption process of Cu(I)–CN by SiO2. However, a similar copper removal capability and low selectivity was observed when Cu(I)–CN–PB was employed as the copper source. Therefore, a modification on the silica’s surface with phenyl groups was performed, in order to enhance the metallic ion selectivity. IR spectroscopy and TGA/DTA analysis confirmed the coupling of organic groups; on the other hand, nitrogen adsorption indicated a decrease on the BET surface area of the silica at 76 %, a modification of the silica structure was observed with the formation of two pore diameter (3.6 and 5.37 nm); 13C CP-MAS NMR indicated two different chemical shifts that corresponded to the phenyl groups on the two different pores observed. Phenyl groups enhance the selectivity for copper in the cyanide effluent, increasing the removal to 99 %.








Similar content being viewed by others
References
Tewodros A, Zhimin T (2012) Biocompatibility of mesoporous silica nanoparticles. Chem Res Toxicol 25:2265–2284
Barton Thomas J, Bull Lucy M, Klemperer Walter G, Loy Douglas A, Brian M, Makoto M, Monson Peter A, Guido P, Scherer George W, Vartuli James C, Yaghi Omar M (1999) Tailored porous materials. Chem Mater 11:2633–2656
Lee JS, Deorkar NV, Tavlarides LL (1998) Adsorption of copper cyanide on chemically active adsorbents. Ind Eng Chem Res 37:2812–2820
Yeoh F-Y, Matsumoto A, Baba T (2009) Facile synthesis of sulfo-functionalized mesoporous silica with strong acidity by temperature-controlled calcination. J Porous Mater 16:283–289
Iqbal G (2001) Bio-doped Nanocomposite Polymers: sol–gel bioencapsulates. Chem Mater 13:3404–3421
Martin H (2005) Ordered mesoporous materials for bioadsorption and biocatalysis. Chem Mater 17:4577–4593
Hartmann S, Brandhuber D, Hüsing N (2007) Glycol-modified silanes: novel possibilities for the synthesis of hierarchically organized (Hybrid) porous materials. Acc Chem Res 40:885–894
Daniel NT, Kenneth JB Jr (2011) Perspective of recent progress in immobilization of enzymes. Catalysis 1:956–968
Jal PK, Patel S, Mishra BK (2004) Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 62:1005–1028
Hernández-Ramirez O, Holmes SM (2008) Novel and modified materials for wastewater treatment applications. J Mater Chem 18:1761–2751
Cai M, Xiao R, Yan T, Zhao H (2014) A simple and green synthesis of diaryl sulfides catalyzed by an MCM-41-immobilized copper(I) complex in neat water. J Organomet Chem 749:55–60
Sharp Kenneth G (2005) Star alkoxysilane molecules gels and appreciably tough glasses. J Mater Chem 15:3812–3820
Hench Larry L, West JK (1990) The sol–gel process. Chem Rev 90:33–72
Sahoo SK (1991) Extractive-chromatographic separation of bismuth with aliquat 336S from citrate solutions. Talanta 38:789–792
Hu Z, Zhang X, Zhang D, Wang J-X (2012) Adsorption of Cu2+ on amine-functionalized mesoporous silica brackets. Water Air Soil Pollut 223:2743–2749
Awual MR, Rahman IMM, Yaita T, Khaleque MD, Ferdows M (2014) pH dependent Cu(II) and Pd(II) ions detection and removal from aqueous media by an efficient mesoporous adsorbent. Chem Eng J 236:100–109
Wang Z, Wu G, Wang M, He Ch (2009) An imprinted organic-inorganic hybrid sorbent for selective separation of copper ion from aqueous solution. J Mater Sci 44:2694–2699. doi:10.1007/s10853-009-3353-7
Mattigod SV, Fryxell GE, Parker KE (2007) Anion binding in self-assembled monolayers in mesoporous supports (SAMMS). Inorg Chem Commun 10:646–648
Fryxell GE, Hauser TA, Nie Z, Ferris KF, Mattigod S, Gong M, Hallen RT (1999) Desing and synthesis of selective mesoporous anion traps. Chem Mater 11:2148–2154
Da’na E, Sayari A (2012) Adsorption of heavy metals on amine-funtionalized SBA-15 prepared by co-condensation: applications to real water samples. Desalination 285:62–67
Barczak M, Oszust M, Michalak K, Gdula K, Pasieczna-Patkowska S, Zieba E, Dabrowski A (2014) Functionalized SBA-15 organosilicas as sorbents of mercury(II), cadmium(II) and copper(II). Glass Phys Chem 40:41–48
Behbahani M, Najafi F, Amini MM, Sadeghi O, Begheri A, Hassanlou PG (2014) Solid phase extraction using nanoporous MCM-41 modified with 3,4-dihydroxybenzaldehyde for simultaneous preconcentration and removal of gold(III), palladium(II), copper(II)and silver(I). J Ind Eng Chem 20:2248–2255
Behbahani M, Najafi M, Amini MM, Sadeghi O, Begheri A, Salarian M (2013) Dithizone-modified nanoporous fructose as a novel sorbent for solid-phase extraction of ultra-trace levels of heavy metals. Microchim Acta 180:911–920
Begheri A, Behbahani M, Amini MM, Sadeghi O, Taghizade M, Baghayi L, Salarian M (2012) Simultaneous separation and determination of trace amounts of Cd(II) and Cu(II) in environmental samples using novel diphenylcarbazide modified nanoporous silica. Talanta 89:455–461
Ebrahimzadeh H, Behbahani M, Yamini Y, Adinasab L, Asgharinezhad AA (2013) Optimization of Cu(II)-ion imprinted nanoparticles for trace monitoring of copper in water and fish samples using a box-Behnken design. React Funct Polym 73:23–29
Awual MR, Ismael M, Khaleque MA, Yaita T (2014) Ulta-trace copper(II) detection and removal from wastewater using novel meso-adsorbent. J Ind Eng Chem 20:2332–2340
Awual MR, Yaita T, Okamoto Y (2014) A novel ligand based dual conjugate adsorbent for cobalt(II) and copper(II) ions capturing from water. Sens Actuators B 203:71–80
Awual MR, Yaita T, El-Safty SA, Shiwaku H, Suzuki S, Okamoto Y (2013) Copper(II) ions capturing from water using ligand modified a new type mesoporous adsorbent. Chem Eng J 221:322–330
Wang Q, Gao W, Liu Y, Yuan J, Zhijun Xu, Zeng Q, Yuguang Li, Schröder M (2014) Simultaneous adsorption of Cu(II) and SO4 2− ions by a novel silica gel funtionalized with a ditopic zwitterionic Schiff base ligand. Chem Eng J 250:55–65
Dai X, Simons A, Breuer P (2012) A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores. Miner Eng 25:1–13
Alonso-González O, Nava-Alonso F, Uribe-Salas A (2009) Copper removal from cyanide solutions by acidification. Miner Eng 22:324–329
Dai X, Breuer PL (2009) Cyanide and copper cyanide recovery by activated carbon. Miner Eng 22:469–476
Muir DM (2011) A review of the selective leaching of gold from oxidized copper–gold ores with ammonia–cyanide and new insights for plant control and operation. Miner Eng 24:576–582
Xie F, Dreisinger DB (2010) Copper solvent extraction from alkaline cyanide solution with guanidine extractant LIX 7950. Trans Nonferrous Met Soc China 20:1136–1140
Mokhonoana MP, Coville NJ (2010) Synthesis of [Si]-MCM-41 from TEOS and water glass: the water glass-enhanced condensation of TEOS under alkaline conditions. J Sol-Gel Sci Technol 54:83–92
Salazar-Hernández M, Salazar-Hernández C, Gutiérrez-Fuentes A, Elorza E, Carrera-Rodríguez M, Puy-Alquiza MJ (2014) Silica from rice husks employed as drug delivery for folic acid. J Sol-Gel Sci Technol 71:514–521
Salazar-Hernández C, Zarraga R, Alonso S, Sugita S, Calixto S, Cervantes J (2009) Effect of solvent type on polycondensation of TEOS catalyzed by DBTL as used for stone consolidation. J Sol-Gel Sci Technol 49:301–310
Zhuravlev LT (2000) The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf A 173:1–38
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem Mater 13:3169–3183
Foo KY, Hameed BH (2010) Insigts into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Ijagbemi ChO, Baek M, Kim D (2009) Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater 166:538–546
Acknowledgements
The authors wish to acknowledge the financial support of SEP-PROMEP (IDCA 7168, UGTO-CA-116 and F-PROMEP-39/Rev-03). They also acknowledge Dra. Fabiola C. Nava Alonso and Dr. Gerardo González for their support in the characterization of the sample.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Salazar-Hernández, M.M., Salazar-Hernández, C., Elorza-Rodríguez, E. et al. The use of mesoporous silica in the removal of Cu(I) from the cyanidation process. J Mater Sci 50, 439–446 (2015). https://doi.org/10.1007/s10853-014-8603-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8603-7