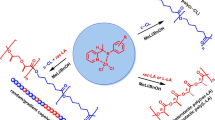
Abstract
In the twenty-first century, one of the central focus of polymer research in academia and industries is directed towards the design of environmentally-benign materials produced from reagents that have minimal deleterious effects on our environment. The aliphatic polyester PLA is one such example. Due to its biodegradable, biorenewable and biocompatible nature, PLA finds diverse applications, especially in the biomedical field. PLA is exclusively synthesized by the ring-opening polymerization of lactide (cyclic dimer of lactic acid) in the presence of a catalyst. The macrocycles and macrocyclic metal moieties can act as effective catalysts for the polymerization resulting in the formation of PLA with controlled tacticity and predetermined molecular weight. This review reports metal-based catalytic systems supported by porphyrin, calixarene and bispyrrolidine- salan as ancillary ligand and metal-free organocatalyst sparteine for the ROP of LA. The variation in catalytic activity, tacticity of PLA, and PLA's molecular weight distribution by substitutional changes in the catalyst framework have been discussed in detail.
Graphic abstract






















































Similar content being viewed by others
Change history
11 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10847-021-01060-y
Abbreviations
- ROP:
-
Ring Opening Polymerization
- LA:
-
Lactide
- PLA:
-
Polylactide
- PGA:
-
Polyglycolide
- PLGA:
-
Poly Lactic-co-Glycolic-acid
- PCL:
-
Polycaprolactone
- PDI:
-
Polydispersity index
- TPPH2 :
-
Tetraphenylporphyrin
- PO:
-
Propylene oxide
- PPO:
-
Polypropylene oxide
- PPN+Cl− :
-
Bis(triphenylphosphine) iminium chloride
- CHO:
-
Cyclohexene oxide
References
Chen, G.Q., Patel, M.K.: Plastics derived from biological sources: present and future: a technical and environmental review. Chem. Rev. 112, 2082–2099 (2012)
Dechy-Cabaret, O., Martin-Vaca, B., Bourissou, D.: Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 104, 6147–6176 (2004)
Rhim, J.W., Park, H.M., Ha, C.S.: Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 38, 1629–1652 (2013)
Hayashi, T.: Biodegradable polymers for biomedical uses. Prog. Polym. Sci. 19, 663–702 (1994)
Chiellini, E., Solaro, R.: Biodegradable polymeric materials. Adv. Mater. 8, 305–313 (1996)
Ikada, Y., Tsuji, H.: Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 21, 117–132 (2000)
Sinclair, R.G.: The case for polylactic acid as a commodity packaging plastic. J Mass Spec-Pure Appl Chem 33, 585–597 (1996)
Ahmed, J., Varshney, S.K.: Polylactides—chemistry, properties and green packaging technology: a review. Int. J. Food Prop. 14, 37–58 (2011)
Bogaert, J.C., Coszach, P.: Poly (lactic acids): a potential solution to plastic waste dilemma. Macromol. Symp. 153, 287–303 (2000)
Cheng, Y., Deng, S., Chen, P., Ruan, R.: Polylactic acid (PLA) synthesis and modifications: a review. Front Chem China 4, 259–264 (2009)
Hu, Y., Daoud, W.A., Cheuk, K.K.L., Lin, C.S.K.: Newly developed techniques on polycondensation, ring-opening polymerization and polymer modification: Focus on poly (lactic acid). Materials 9, 133 (2016)
Stanford, M.J., Dove, A.P.: Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 39, 486–494 (2010)
Sarazin, Y., Carpentier, J.F.: Discrete cationic complexes for ring-opening polymerization catalysis of cyclic esters and epoxides. Chem. Rev. 115, 3564–3614 (2015)
Dagorne, S., Fliedel, C.: Organoaluminum species in homogeneous polymerization catalysis. Top. Organomet. Chem. 41, 125–172 (2013)
Ghosh, S., Gowda, R.R., Jagan, R., Chakraborty, D.: Gallium and indium complexes containing the bis (imino) phenoxide ligand: synthesis, structural characterization and polymerization studies. Dalton Trans. 44, 10410–10422 (2015)
Mandal, M., Monkowius, U., Chakraborty, D.: Synthesis and structural characterization of titanium and zirconium complexes containing half-salen ligands as catalysts for polymerization reactions. New J. Chem. 40, 9824–9839 (2016)
O’Keefe, B.J., Hillmyer, M.A., Tolman, W.B.: Polymerization of lactide and related cyclic esters by discrete metal complexes. J. Chem. Soc. Dalton Trans. 15, 2215–2224 (2001)
Nederberg, F., Connor, E.F., Möller, M., Glauser, T., Hedrick, J.L.: New paradigms for organic catalysts: the first organocatalytic living polymerization. Angew. Chem. Int. Ed. 40, 2712–2715 (2001)
Zhang, L., Nederberg, F., Messman, J.M., Pratt, R.C., Hedrick, J.L., Wade, C.G.: Organocatalytic stereoselective ring-opening polymerization of lactide with dimeric phosphazene bases. J. Am. Chem. Soc. 129, 12610–12611 (2007)
Hormnirun, P., Marshall, E.L., Gibson, V.C., Pugh, R.I., White, A.J.P.: Study of ligand substituent effects on the rate and stereoselectivity of lactide polymerization using aluminumsalen-type initiators. Proc. Natl. Acad. Sci. U. S. A. 103, 15343–15348 (2006)
Qian, F., Liu, K., Ma, H.: Amidinate aluminium complexes: synthesis, characterization and ring-opening polymerization of rac-lactide. Dalton Trans. 39, 8071–8083 (2010)
Stasiw, D.E., Luke, A.M., Rosen, T., League, A.B., Mandal, M., Neisen, B.D., Cramer, C.J., Kol, M., Tolman, W.B.: Mechanism of the polymerization of rac-lactide by fast zinc alkoxide catalysts. Inorg. Chem. 56, 14366–14372 (2017)
Pedersen, C.J.: Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 89, 2495–2496 (1967)
Cram, D.J., Cram, J.M.: Host-guest chemistry. Science 183, 803–809 (1974)
Lehn, J.M.: Supramolecular Chemistry: Concepts and Perspectives. VCH, Weinheim (1995)
Battersby, A.R., Fookes, C.J., Matcham, G.W., McDonald, E.: Biosynthesis of the pigments of life: formation of the macrocycle. Nature 285, 17–21 (1980)
Mokhtari, B., Pourabdollah, K., Dallali, N.: A review of calixarene applications in nuclear industries. J. Radioanal. Nucl. Chem. 287, 921–934 (2011)
Nimse, S.B., Kim, T.: Biological applications of functionalized calixarenes. Chem. Soc. Rev. 42, 366–386 (2013)
Redshaw, C.: Coordination chemistry of the larger calixarenes. Coord. Chem. Rev. 244, 45–70 (2003)
O’Keefe, B.J., Hillmyer, M.A., Tolman, W.B.: Polymerization of lactide and related cyclic esters by discrete metal complexes. J. Chem. Soc. Dalton Trans. (2001). https://doi.org/10.1039/b104197p
Amgoune, A., Thomas, C.M., Carpentier, J.F.: Controlled ring-opening polymerization of lactide by group 3 metal complexes. Pure Appl. Chem. 79, 2013–2030 (2007)
Dos Santos Vieira, I., Herres-Pawlis, S.: Lactide Polymerisation with Complexes of Neutral N-Donors–New Strategies for Robust Catalysts. Eur. J. Inorg. Chem. 2012, 765–774 (2012)
Kremer, A.B., Mehrkhodavandi, P.: Dinuclear catalysts for the ring opening polymerization of lactide. Coord. Chem. Rev. 380, 35–57 (2019)
Sauer, A., Kapelski, A., Fliedel, C., Dagorne, S., Kol, M., Okuda, J.: Structurally well-defined group 4 metal complexes as initiators for the ring-opening polymerization of lactide monomers. Dalton Trans. 42, 9007–9023 (2013)
Wheaton, C.A., Hayes, P.G.: Designing cationic zinc and magnesium catalysts for coordination–insertion polymerization of lactide. Comments Inorg. Chem. 32, 127–162 (2011)
Chisholm, M.H.: Concerning the ring-opening polymerization of lactide and cyclic esters by coordination metal catalysts. Pure Appl. Chem. 82, 1647–1662 (2010)
Osten, K.M., Mehrkhodavandi, P.: Indium catalysts for ring opening polymerization: exploring the importance of catalyst aggregation. Acc. Chem. Res. 50, 2861–2869 (2017)
Sergeeva, E., Kopilov, J., Goldberg, I., Kol, M.: 2, 2′-Bipyrrolidine versus 1, 2-Diaminocyclohexane as Chiral Cores for Helically Wrapping Diamine−Diolate Ligands. Inorg. Chem. 48, 8075–8077 (2009)
Jiang, Z., Zhao, J., Zhang, G.: Ionic organocatalyst with a urea anion and Tetra-n-butyl ammonium cation for rapid, selective, and versatile ring-opening polymerization of lactide. ACS Macro Lett. 8, 759–765 (2019)
Engel, J., Cordellier, A., Huang, L., Kara, S.: Enzymatic Ring-Opening Polymerization of Lactones: Traditional Approaches and Alternative Strategies. ChemCatChem 11, 4983–4997 (2019)
Kamber, N.E., Jeong, W., Waymouth, R.M., Pratt, R.C., Lohmeijer, B.G.G., Hedrick, J.L.: Organocatalytic ring-opening polymerization. Chem. Rev. 107, 5813–5840 (2007)
Mezzasalma, L., Dove, A.P., Coulembier, O.: Organocatalytic ring-opening polymerization of L-lactide in bulk: A long standing challenge. Eur. Polym. J. 95, 628–634 (2017)
Pothupitiya, J.U., Dharmaratne, N.U., Jouaneh, T.M.M., Fastnacht, K.V., Coderre, D.N., Kiesewetter, M.K.: H-Bonding Organocatalysts for the Living, Solvent-Free Ring-Opening Polymerization of Lactones: Toward an All-Lactones. All-Conditions Approach. Macromolecules 50, 8948–8954 (2017)
Lin, B., Waymouth, R.M.: Organic ring-opening polymerization catalysts: reactivity control by balancing acidity. Macromolecules 51, 2932–2938 (2018)
Ottou, W.N., Sardon, H., Mecerreyes, D., Vignolle, J., Taton, D.: Update and challenges in organo-mediated polymerization reactions. Prog. Polym. Sci. 56, 64–115 (2016)
Zhang, D., Zi, G.: N-heterocyclic carbene (NHC) complexes of group 4 transition metals. Chem. Soc. Rev. 44, 1898–1921 (2015)
Wink, M., Meißner, C., Witte, L.: Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry 38, 139–153 (1995)
Tempelaar, S., Mespouille, L., Dubois, P., Dove, A.P.: Organocatalytic synthesis and postpolymerization functionalization of allyl-functional poly (carbonate) s. Macromolecules 44, 2084–2091 (2011)
Mostovaya, O.A., Gorbachuk, V.V., Padnya, P.L., Vivilova, A.A., Evtugyn, G.A., Stoikov, I.I.: Modification of oligo- and polylactides with macrocyclic fragments: synthesis and properties, p. 7. Front, Chem (2019)
Gorbachuk, V.V., Padnya, P.L., Mostovaya, O.A., Gerasimov, A.V., Stoikov, I.I.: Towards novel functional polymers: Ring-opening polymerization of L-lactide with p-tert-butylthiacalix[4]arene derivatives. React. Funct. Polym. 150, 104546 (2020)
Dubois, P., Jacobs, C., Jérôme, R., Teyssie, P.: Macromolecular engineering of polylactones and polylactides. 4. Mechanism and kinetics of lactide homopolymerization by aluminumisopropoxide. Macromolecules 24, 2266–2270 (1991)
Imran, M., Ramzan, M., Qureshi, A.K., Khan, M.A., Tariq, M.: Emerging applications of porphyrins and metalloporphyrins in biomedicine and diagnostic magnetic resonance imaging. Biosensors 8, 95 (2018)
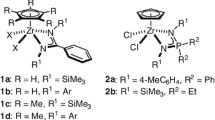
Trofimoff, L., Aida, T., Inoue, S.: Formation of poly (lactide) with controlled molecular weight. Polymerization of lactide by aluminum porphyrin. Chem. Lett. 16, 991–994 (1987)
Li, D., Gao, B., Duan, Q.: Syntheses of biodegradable and biorenewable polylactides initiated by aluminum complexes bearing porphyrin derivatives by the ring-opening polymerization of lactides. J Biomat Sci-Polym E 30, 846–860 (2019)
Balasanthiran, V., Chatterjee, C., Chisholm, M.H., Harrold, N.D., RajanBabu, T.V., Warren, G.A.: Coupling of propylene oxide and lactide at a porphyrin chromium (III) center. J. Am. Chem. Soc. 137, 1786–1789 (2015)
Summerville, D.A., Jones, R.D., Hoffman, B.M., Basolo, F.: Chromium (III) porphyrins. Chemical and spectroscopic properties of chloro-meso-tetraphenylporphinatochromium (III) in nonaqueous solutions. J. Am. Chem. Soc. 99, 8195–8202 (1977)
Chatterjee, C., Chisholm, M.H.: Ring-Opening Polymerization Reactions of Propylene Oxide Catalyzed by Porphyrin Metal (3+) Complexes of Aluminum. Chromium and Cobalt. Chem. Rec. 13, 549–560 (2013)
Anker, M., Balasanthiran, C., Balasanthiran, V., Chisholm, M.H., Jayaraj, S., Mathieu, K., Piromjitpong, P., Praban, P., Raya, B., Simonsick, W.J.: A new route for the preparation of enriched iso-polylactide from rac-lactide via a Lewis acid catalyzed ring-opening of an epoxide. Dalton Trans. 46, 5938–5945 (2017)
Praban, S., Piromjitpong, P., Balasanthiran, V., Jayaraj, S., Chisholm, M.H., Tantirungrotechai, J., Phomphrai, K.: Highly efficient metal (III) porphyrin and salen complexes for the polymerization of rac-lactide under ambient conditions. Dalton Trans. 48, 3223–3230 (2019)
Li, D., Gao, B., Duan, Q.: Preparation of star-shaped functionalized polylactides by metal porphyrin complexes as both catalysts and cocatalysts. J. Porphyrins Phthalocyanines 23, 1020–1027 (2019)
Li, Y., Zhao, K.Q., Redshaw, C., Ortega, B.A.M., Nuñez, A.Y., Hanna, T.A.: Coordination chemistry and applications of phenolic calixarene–metal complexes. PATAI’S Chemistry of Functional Groups (2009). https://doi.org/10.1002/9780470682531.pat0616
Bukhaltsev, E., Frish, L., Cohen, Y., Vigalok, A.: Single-site catalysis by bimetallic zinc calixarene inclusion complexes. Org. Lett. 7, 5123–5126 (2005)
Frediani, M., Sémeril, D., Mariotti, A., Rosi, L., Frediani, P., Rosi, L., Matt, D., Toupet, L.: Ring Opening Polymerization of Lactide under Solvent-Free Conditions Catalyzed by a Chloro titanium Calix [4] arene Complex. Macromol. Rapid Commun. 29, 1554–1560 (2008)
Frediani, M., Sémeril, D., Matt, D., Rosi, L., Frediani, P., Rizzolo, F., Papini, A.M.: Ring-opening polymerisation of rac-lactide using a calix 4 arene-based titanium (IV) complex. Int J. Polym. Sci (2010). https://doi.org/10.1155/2010/490724
Walton, M.J., Lancaster, S.J., Redshaw, C.: Highly Selective and Immortal Magnesium Calixarene Complexes for the Ring-Opening Polymerization of rac-Lactide. ChemCatChem 6, 1892–1898 (2014)
Mayilmurugan, R., Traar, P., Schachner, J.A., Volpe, M., Mösch-Zanetti, N.C.: Dioxidomolybdenum (VI) Complexes Containing Ligands with the Bipyrrolidine Backbone as Efficient Catalysts for Olefin Epoxidation. Eur. J. Inorg. Chem. 2013, 3664–3670 (2013)
Hador, R., Botta, A., Venditto, V., Lipstman, S., Goldberg, I., Kol, M.: The Dual-Stereocontrol Mechanism: Heteroselective Polymerization of rac-Lactide and Syndioselective Polymerization of meso-Lactide by Chiral AluminumSalan Catalysts. Angew. Chem. Int. Ed. 58, 14679–14685 (2019)
Jones, M.D., Hancock, S.L., McKeown, P., Schäfer, P.M., Buchard, A., Thomas, L.H., Lowe, J.P.: Zirconium complexes of bipyrrolidine derived salan ligands for the isoselective polymerisation of rac-lactide. Chem. Commun. 50, 15967–15970 (2014)
Kaminsky, W., Arndt, M.: 17. Mechanism of the First Steps of the Isotactic Polymerization with Metallocene Catalysts. In: Kaminsky, W. (ed.) Studies in Surface Science and Catalysis, vol. 89, pp. 179–192. Elsevier, Amsterdam (1994)
Jones, M.D., Brady, L., McKeown, P., Buchard, A., Schäfer, P.M., Thomas, L.H., Lowe, J.P.: Metal influence on the iso-and hetero-selectivity of complexes of bipyrrolidine derived salan ligands for the polymerisation of rac-lactide. Chem. Sci. 6, 5034–5039 (2015)
Addison, A.W., Rao, T.N., Reedijk, J., van Rijn, J., Verschoor, G.C.: Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2′-yl)-2, 6-dithiaheptane] copper (II) perchlorate. J. Chem. Soc. Dalton Trans. (1984). https://doi.org/10.1039/DT9840001349
Yang, L., Powell, D.R., Houser, R.P.: Structural variation in copper (I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index, τ 4. Dalton Trans (2007). https://doi.org/10.1039/B617136B
Quilter, H.C., Drewitt, R.H., Mahon, M.F., Kociok-Köhn, G., Jones, M.D.: Synthesis of Li (I), Zn (II) and Mg (II) complexes of amine bis (phenolates) and their exploitation for the ring opening polymerisation of rac-lactide. J. Organomet. Chem. 848, 325–331 (2017)
Beament, J., Kociok-Köhn, G., Jones, M.D., Buchard, A.: Bipyrrolidinesalan alkoxide complexes of lanthanides: synthesis, characterisation, activity in the polymerisation of lactide and mechanistic investigation by DOSY NMR. Dalton Trans. 47, 9164–9172 (2018)
Beament, J., Mahon, M.F., Buchard, A., Jones, M.D.: Salan group 13 complexes–structural study and lactide polymerisation. New J. Chem. 41, 2198–2203 (2017)
Press, K., Goldberg, I., Kol, M.: Mechanistic insight into the stereochemical control of lactide polymerization by salan–aluminum catalysts. Angew. Chem. Int. Ed. 54, 14858–14861 (2015)
Beament, J., Mahon, M.F., Buchard, A., Jones, M.D.: Aluminum complexes of monopyrrolidine ligands for the controlled ring-opening polymerization of lactide. Organometallics 37, 1719–1724 (2018)
Villalpando-Vargas, F., Medina-Ceja, L.: Sparteine as an anticonvulsant drug: Evidence and possible mechanism of action. Seizure 39, 49–55 (2016)
Chen, S., Liu, Y., Li, Z., Wang, X., Dong, H., Sun, H., Yang, K., Gebru, H., Guo, K.: H-bonding binary organocatalysis promoted amine-initiated ring-opening polymerizations of lactide from polysarcosine to diblock copolymers. Eur. Polym. J. 97, 389–396 (2017)
Liu, J., Chen, C., Li, Z., Wu, W., Zhi, X., Zhang, Q., Wu, H., Wang, X., Cui, S., Guo, K.: A squaramide and tertiary amine: an excellent hydrogen-bonding pair organocatalyst for living polymerization. Polym. Chem. 6, 3754–3757 (2015)
Pratt, R.C., Lohmeijer, B.G.G., Long, D.A., Lundberg, P.N.P., Dove, A.P., Li, H., Wade, C.G., Waymouth, R.M., Hedrick, J.L.: Exploration, Optimization, and Application of Supramolecular Thiourea−Amine Catalysts for the Synthesis of Lactide (Co) polymers. Macromolecules 39, 7863–7871 (2006)
Coulembier, O., De Winter, J., Josse, T., Mespouille, L., Gerbaux, P., Dubois, P.: One-step synthesis of polylactide macrocycles from sparteine-initiated ROP. Polym. Chem. 5, 2103–2108 (2014)
Thomas, C., Milet, A., Peruch, F., Bibal, B.: Activation of carbonyl bonds by quaternary ammoniums and a (Na+: crown-ether) complex: investigation of the ring-opening polymerization of cyclic esters. Polym. Chem. 4, 3491–3498 (2013)
Eisenreich, F., Kathan, M., Dallmann, A., Ihrig, S.P., Schwaar, T., Schmidt, B.M., Hecht, S.: A photoswitchable catalyst system for remote-controlled (co) polymerization in situ. Nat. Catal. 1, 516–522 (2018)
Zhang, D., Jardel, D., Peruch, F., Calin, N., Dufaud, V., Dutasta, J.P., Martinez, A., Bibal, B.: Azaphosphatranes as Hydrogen-Bonding Organocatalysts for the Activation of Carbonyl Groups: Investigation of Lactide Ring-Opening Polymerization. Eur. J. Org. Chem. 2016, 1619–1624 (2016)
Funding
Funding was provided by UGC-ISF Grant No. 6-2/2018(IC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access cancellation.
Rights and permissions
About this article
Cite this article
Roy, S.S., Sarkar, S. & Chakraborty, D. Macrocycles in dual role: ancillary ligands in metal complexes and organocatalysts for the ring-opening polymerization of lactide. J Incl Phenom Macrocycl Chem 100, 1–36 (2021). https://doi.org/10.1007/s10847-021-01045-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-021-01045-x