Abstract
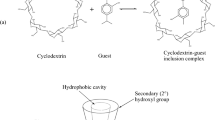
The study focuses on the formation of inclusion complexes of indole chalcone (IC) derivatives with β-cyclodextrin (β-CD), which involves absorption and steady state fluorescence spectroscopies. The formation of inclusion complexes is validated by increase in their absorbance and fluorescence intensity as well as the blue shift with increase in the concentration of β-CD in the aqueous solution. The stoichiometries and binding constants (Kin) of these complexes have been investigated by monitoring their absorbance and fluorescence spectral profiles. The data are analyzed by Benesi–Hildebrand plots as well as Job’s method, which indicate 1:1 stoichiometry of IC:β-CD complexes. Fluorescence measurements are also used to investigate the effect of temperature on the stability of inclusion complexes. Stability of IC:β-CD complexes is significantly affected with variation in substituents on the phenyl ring and temperature. It is observed that the stability of the inclusion complex decreases with increase in temperature; Kin(293 K) > Kin(298 K) > Kin(308 K) > Kin(318 K). All the experimental results and the geometrical data obtained using PM3 semiempirical method illustrate the partial inclusion of IC derivatives from the phenyl ring side in β-CD cavity. The binding process of IC derivatives with β-CD is found to be exothermic in nature and seems to be controlled by electrostatic and hydrophobic forces. The binding free energies calculated using semiemprical PM3 method for IC:β-CD complexes are found to be in the order: I < OH–I < Me–I < OMe–I < NH2–I, which largely supports the findings based on the experimental binding constants.








Similar content being viewed by others
References
Maria, K., Dimitra, H.L., Maria, G.: Synthesis and anti-inflammatory activity of chalcones and related mannich bases. Med. Chem. 4(6), 586–596 (2008)
Cocconcelli, G., Diodato, E., Caricasole, A., Gaviraghi, G., Genesio, E., Ghiron C., Magnoni, L., Pecchioli, E., Plazzib, P.V., Terstappen, G.C.: Aryl azoles with neuroprotective activity-parallel synthesis and attempts at target identification. Bioorg. Med. Chem. 16(4), 2043–2052 (2008)
Budakoti, A., Bhat, A.R., Athar, F., Azam, A.: Syntheses and evaluation of 3-(3-bromo phenyl)-5-phenyl-1-(thiazolo [4,5-b] quinoxaline-2-yl)-2-pyrazoline derivatives. Eur. J. Med. Chem. 43(8), 1749–1757 (2008)
Kumar, D., Kumar, N.M., Akamatsu, K., Kusaka, E., Harada, H., Ito, T.: Synthesis and biological evaluation of indolyl chalcones as antitumor agents. Bioorg. Med. Chem. Lett. 20(13), 3916–3919 (2010)
Saroj, M.K., Sharma, N., Rastogi, R.C.: Solvent effect profiles of absorbance and fluorescence spectra of some indole based chalcones. J. Fluoresc. 21, 2213–2227 (2011)
Saroj, M.K., Sharma, N., Rastogi, R.C.: Photophysical study of some 3-benzoylmethyleneindol-2-ones and estimation of ground and excited states dipole moments from solvatochromic methods using solvent polarity parameters. J. Mol. Struct. 1012, 73–86 (2012)
López, S., Castelli, M., Zacchino, S., Domínguez, J., Lobo, G., Charris-Charris, J., Cortés, J., Ribas, J., Devia, C., Rodríguez, A.: In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 9, 1999–2013 (2001)
Singh, P., Anand, A., Kumar, V.: Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem. 85, 758–777 (2014)
Gill, K.K., Kaddoumi, A., Nazzal, S.: PEG-lipid micelles as drug carriers: physiochemical attributes, formulation principles and biological implication. J. Drug Target. 23, 222–231 (2015)
Kuo, Y.-C., Lin, C.-C.: Rescuing apoptotic neurons in alzheimer’s disease using wheat germ agglutinin-conjugated and cardiolipin-conjugated liposomes with encapsulated nerve growth factor and curcumin. Int. J. Nanomed. 10, 2653–2672 (2015)
Lao, W., Song, C., You, J., Ou, Q.: Fluorescence and β-cyclodextrin inclusion properties of three carbazole-based dyes. Dyes Pigments. 95(3), 619–626 (2012)
Hazra, S., Hossain, M., Kumar, G.S.: Studies on α-, β-, and γ-cyclodextrin inclusion complexes of isoquinoline alkaloids berberine, palmatine and coralyne. J. Incl. Phenom. Macrocycl. Chem. 78(1–4), 311–323 (2014)
Li, J., Zhang, H., Yan, Y., Sun, S.: Study of the inclusion complex and antioxidating activity of Wogonin with b-cyclodextrin and hydroxypropylcyclodextrin. J. Inclus. Phenom. Macrocycl. Chem. 84(1–2), 115–120 (2016)
Guzzo, T., Mandaliti, W., Nepravishta, R., Aramini, A., Bodo, E., Daidone, I., Allegretti, M., Topai, A., Paci, M.: Conformational change in the mechanism of inclusion of ketoprofen in β-cyclodextrin: NMR Spectroscopy, Ab Initio calculations, molecular dynamics simulation and photoreactivity. J. Phys. Chem. B 120(41), 10668–10678 (2016)
Rajamohan, R., Nayaki, K., Swaminathan, S.M.: Photophysical and photoprototropic characteristics of 2-aminobenzothiazole in β-cyclodextrin medium. J. Fluoresc. 27(2), 689–699 (2017)
Wang, Q.Q., Day, V.W., Bowman-James, K.: Chemistry and structure of a host-guest relationship: the power of NMR and X-ray diffraction in tandem. J. Am. Chem. Soc. 135(1), 392–399 (2013)
Guo, X., Jia, X., Du, J., Xiao, L., Li, F., Liao, L., Liu, L.: Host-guest chemistry of cyclodextrin carbamates and cellulose derivatives in aqueous solution. Carbohyd. Polym. 98(1), 982–987 (2013)
Kim, T.K., Yoo, H.H.: Anticancer effect of docetaxel/hydroxypropyl-beta-cyclodextrin complex without histamine release. J. Incl. Phenom. Macrocycl. 83(3–4), 355–361 (2015)
Lutfor, M.R., Hegde, G., Kumar, S., Tschierske, C., Chigrinov, V.G.: Synthesis and characterization of bent-shaped azobenzene monomers: guest-host effects in liquid crystals with azo dyes for optical image storage devices. Opt. Mater. 32(1), 176–183 (2009)
Haino, T.: Supramolecular chemistry: from host-guest complexes to supramolecular polymers. Yuki Gosei Kagaku Kyokaishi 71(11), 1172–1181 (2013)
Fan, H., Xing, R., Wang, X., Xu, Y., Wang, Q., He, P., Fang, Y.: A host-guest-recognition-based electrochemical sensor for sequence-specific DNA detection. Electroanalysis 22(15), 1781–1786 (2010)
Jankowska, K.I., Pagba, C.V., Piatnitski Chekler, E.L., Deshayes, K., Piotrowiak, P.: Electrostatic docking of a supramolecular host-guest assembly to Cytochrome C probed by bidirectional photoinduced electron transfer. J. Am. Chem. Soc. 132(46), 16423–16431 (2010)
Rajamohan, R., Nayaki, S.K., Sivakumar, K., Swaminathan, M.: Photophysical and photoprototropic characteristics of phenothiazine in aqueous and β-cyclodextrin media. J. Lumin. 168, 245–255 (2015)
Singh, S., Negi, J.S., Bisht, R., Negi, V., Kasliwal, N., Thakur, V., Upadhyay, A.: Development and evaluation of orodispersible sustained release formulation of amisulpride-g-cyclodextrin inclusion complex. J. Incl. Phenom. Macrocycl. Chem. 78(1–4), 239–247 (2014)
Tran, C.D., Fendler, J.H.: Photophysical investigations of chiral amine guest-cyclodextrin host interactions and diastereomeric recognition. J. Phys. Chem. 88(10), 2167–2173 (1984)
Muthu, V.E., Rajamohan, R., Swaminathan, M.: Fluorimetric and prototropic studies on the inclusion complexation of 3,3′-diaminodiphenylsulphone with β-cyclodextrin and its unusual behaviour. Spectrochim. Acta Part A 77(2), 473–477 (2010)
Fontanay, S., Kedzierewicz, F., Duval, R.E., Clarot, I.: Physicochemical and thermodynamic characterization of hydroxy pentacyclic triterpenoic acid/g-cyclodextrin inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 73(1–4), 341–347 (2012)
Samanta, A., Jana, S., Guchhait, N.: Spectral modulation of a charge transfer reaction of 2-methoxy-4-(N,N-dimethylamino)benzaldehyde inside cyclodextrin nanocage. J. Incl. Phenom. Macrocycl. Chem. 75(1–2), 57–68 (2013)
Chandrasekaran, S., Sameena, Y., Israel, V.M., Enoch, V.: Modulation of the interaction of Coumarin 7 with DNA by β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 81(1–2), 225–236 (2015)
Street, K.W. Jr: Cyclodextrin cavity polarity and chromatographic implications. J. Liq. Chromatogr. 10(4), 655–662 (1987)
Gashnga, P.M., Singh, T.S., Mitra, S.: Modulation of ESIPT fluorescence in o-hydroxy acetophenone derivatives: a comparative study in different bio-mimicking aqueous interfaces. J. Mol. Liq. 218, 549–557 (2016)
Varghese, B., Al-Busafi, S.N., Suliman, F.E.O., Al-Kindy, S.M.Z.: Tuning the constrained photophysics of a pyrazoline dye 3-naphthyl-1-phenyl-5-(4-carboxyphenyl)-2-pyrazoline inside the cyclodextrin nanocavities: a detailed insight via experimental and theoretical approach. Spectrochim. Acta A 173, 383–389 (2017)
Benesi, A., Hildebrand, J.H.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71, 2703–2707 (1949)
Roberts, E.L., Chou, P.T., Alexander, T.A., Agbaria, R.A., Warner, I.M.: Effects of organized media on the excited-state intramolecular proton transfer of 10-Hydroxybenzo[h] quinoline. J. Phys. Chem. 99(15), 5431–5437 (1995)
Connors, K.A.: Binding Constants: The Measurements of Molecular Complex Stability. Wiley, New York (1987)
Nigam, S., Durocher, G.: Spectral and photophysical studies of inclusion complexes of some neutral 3H-indoles and their cations and anions with β-cyclodextrin. J. Phys. Chem. 100(17), 7135–7142 (1996)
Munoz de la, P.A., Ndou, T., Zung, J.B., Warner, I.M.: Stoichiometry and formation constants of pyrene inclusion complexes with β- and γ-cyclodextrin. J. Phys. Chem. 95(8), 3330–3334 (1991)
CurveExpert: Professional v1.5.0 documentation © Copyright. Daniel G, Hyams (2011)
Kusumoto, Y.: A spectrofluorimetric method for determining the association constants of pyrene with cyclodextrins based on polarity variation. Chem. Phys. Lett. 136(6), 535–538 (1987)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113–203 (1928)
Stewart, J.J.P.: Optimization of parameters for semiempirical methods I. Method J. Comp. Chem. 10(2), 209–220 (1989)
Stewart, J.J.P.: Optimization of parameters for semiempirical methods III: extension of PM3 to beryllium, magnesium, zinc, gallium, germanium, arsenic, selenium, cadmium, indium, tin, antimony, tellurium, mercury, thallium, lead, and bismuth. J. Comp. Chem. 12(3), 320–341 (1991)
HyperChem: Release 8.0 Professional. Hypercube, Inc., USA (2011)
Addy, P.: Theoretical and Physical Principles of Organic Reactivity, 1 edn. Wiley-Blackwell, New York (1995)
Jaffé, H.H.: A re-examination of the Hammett equation. Chem. Rev. 53(2), 191–261 (1953)
Kano, K., Veno, Y., Hasimoto, S.: Fluorescence studies on the characterization and solubilizing abilities of sodium dodecyl sulfate, hexadecyltrimethyl ammonium chloride and Triton X-100 micelles. J. Phys. Chem. 89(14), 3161–3166 (1985)
He, Y., Shen, X.: Interaction between β-cyclodextrin and ionic liquids in aqueous solutions investigated by a competitive method using a substituted 3H-indole probe. J. Photochem. Photobiol. A 197, 253–259 (2008)
Banica, F.-G.: Chemical Sensors and Biosensors: Fundamentals and Applications. Wiley, Chichester (2012)
Fifere, A., Marangoci, N., Maier, S., Coroaba, A., Maftei, D., Pinteala, M.: Theoretical study on β-cyclodextrin inclusion complexes with propiconazole and protonated propiconazole Beilstein. J. Org. Chem. 8, 2191–2201 (2012)
Li, S., Purd, W.C.: Cyciodextrins and their applications in analytical chemistry. Chemi. Rev. 92(6), 1457–1470 (1992)
Jenitia, M.J., Prabhu, A.A.M., Rajendiran, N.: Theoretical study of inclusion complexation of tricyclic antidepressant drugs with β-cyclodextrin. Indian J. Chem. A. 51(12), 1686–1694 (2012)
Acknowledgements
The financial support from University of Delhi under the Scheme “To strengthen Research and Development Doctoral Research Program” is gratefully acknowledged. Manju K. Saroj is thankful to the University Grants Commission, New Delhi for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saroj, M.K., Payal, R., Jain, S.K. et al. Investigation of indole chalcones encapsulation in β-cyclodextrin: determination of stoichiometry, binding constants and thermodynamic parameters. J Incl Phenom Macrocycl Chem 90, 305–320 (2018). https://doi.org/10.1007/s10847-018-0782-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0782-4