Abstract
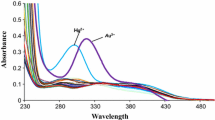
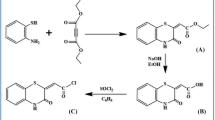
A new calix[4]arene derivative in its cone conformation and bearing Schiff base loop at the lower rim has been synthesized and evaluated as a specific molecular probe for copper ions. The new molecular receptor 4 shows a selective visible change in color from colorless to yellow only in the presence of Cu2+ ions which was confirmed by a significant bathochromic shift (∆λmax = 76 nm) in its absorption spectrum. The stoichiometry of the copper complex was calculated to be 1:1. These results may help to design more efficient chemical sensors for determining copper in biological systems.











Similar content being viewed by others
References
Linder, M.C., Hazegh-Azam, M.: Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 63, 797S–811S (1996)
Uauy, R., Olivares, M., Gonzalez, M.: Essentiality of copper in humans. Am. J. Clin. Nutr. 67, 952S–959S (1998)
de Silva, A.P., Fox, D.B., Huxley, A.J.M., Moody, T.S.: Combining luminescence, coordination and electron transfer for signalling purposes. Coord. Chem. Rev. 205, 41–57 (2000)
Helel, A., Rashid, M.H.O., Choi, C., Kim, H.: New regioisomeric naphthol-substituted thiazole based ratiometric fluorescence sensor for Zn2+ with a remarkable red shift in emission spectra. Tetrahedron 68, 2794–2802 (2012)
Kaur, P., Sareen, D., Singh, K.: Selective colorimetric sensing of Cu2+ using triazolyl monoazo derivative. Talanta 83, 1695–1700 (2011)
Kim, H.J., Hong, J., Hong, A., Ham, S., Lee, J.H., Kim, J.S.: Cu2+-induced Intermolecular static excimer formation of pyrenealkylamine. Org. Lett. 10, 1963–1966 (2008)
Lin, W., Yuan, L., Tan, W., Feng, J., Long, L.: Construction of fluorescent probes via protection/deprotection of functional groups: a ratiometric fluorescent probe for Cu2+. Chem. Eur. J. 15, 1030–1035 (2009)
Gutsche, C.D.: Calixarenes: An Introduction, 2nd edn. Royal Society of Chemistry, Cambridge (2008)
Chawla, H.M., Pant, N., Kumar, S., Kumar, N., Black, D.StC.: Calixarene—based materials for chemical sensors. In: Korotcenkov, G. (ed.) Chemical Sensors Fundamentals of Sensing Materials, vol. 3, p. 300. Momentum Press, New York (2010)
Joseph, R., Rao, C.P.: Ion and molecular recognition by lower rim 1,3-di-conjugates of calix[4]arene as receptors. Chem. Rev. 111, 4658–4702 (2011)
Salmon, L., Thuery, P., Riviere, E., Ephritikhine, M.: Synthesis, structure, and magnetic behaviour of a series of trinuclear Schiff base complexes of 5f (UIV, ThIV) and 3d (CuII, ZnII) ions. Inorg. Chem. 45, 83–93 (2006)
Helel, A., Rashid, M.H.O., Choi, C., Kim, H.: Chromogenic and fluorogenic sensing of Cu2+ based on coumarin. Tetrahedron 67, 2794–2802 (2011)
Chirantan, K., Adhikari, M.D., Datta, B.K., Ramesh, A., Das, G.: A CHEF-based biocompatible turn on ratiometric sensor for sensitiveand selective probing of Cu2+. Sens. Actuators B 188, 1132–1140 (2013)
Aksuner, N., Henden, E., Yilmaz, I.: A highly sensitive and selective fluorescent sensor for the determination of copper(II) based on a Schiff base. Dyes Pigm. 83, 211–217 (2009)
Wang, S., Men, G., Zhao, L.: Binaphthyl-derived salicylidene Schiff base for dual-channel sensing of Cu, Zn cations and integrated molecular logic gates. Sens. Actuators B 145, 826–831 (2010)
Paul, B.K., Kar, S., Guchhait, V.: A Schiff base-derived new model compound for selective fluorescence sensing of Cu(II) and Zn(II) with ratiometric sensing potential: synthesis, photophysics and mechanism of sensory action. J. Photochem. Photobiol. A 220, 153–163 (2011)
Yang, L., Song, Q., Damit-Og, K.: Synthesis and spectral investigation of a Turn-On fluorescence sensor with high affinity to Cu2+. Sens. Actuators B 176, 181–185 (2013)
Chen, Z., Wang, L., Zou, G., Tang, J., Cai, X., Teng, M., Chen, L.: Highly selective fluorescence turn-on chemosensor based on naphthalimide derivatives for detection of copper(II) ions. Spectrochim. Acta A 105, 7–61 (2013)
Iqbal, M., Mangiafico, T., Gutsche, C.D.: Calix[4]arenes 21: the conformations and structures of the products of aroylation of the calix[4]arenes. Tetrahedron 43, 4917–4930 (1987)
Gutsche, C.D., Iqbal, M.: p-tert-Butylcalix[4]arene. Org. Synth. 68, 234–237 (1990)
Gutsche, C.D., Iqbal, M., Steward, D.: Calixarenes. 19. Syntheses procedure for p-tert-butylcalix[4]arene. J. Org. Chem. 51, 742–745 (1986)
Chawla, H.M., Shrivastava, R., Sahu, S.N.: A new class of functionalized calix[4]arenes as neutral receptors for colorimetric detection of fluoride ions. New J. Chem. 32, 1999–2005 (2008)
Bao, X., Yu, J., Zhou, Y.: Selective colorimetric sensing for F- by a cleft shaped anion receptor containing amide and hydroxyl as recognition units. Sens. Actuators B 140, 467–472 (2009)
Mashraqui, S.H., Ghorpade, S.S., Tripathi, S., Britto, S.: A new indole incorporated chemosensor exhibiting selective colorimetric and fluorescence ratiometric signaling of fluoride. Tetrahedron Lett. 53, 765–768 (2012)
Wu, S.-P., Du, K.-J., Sung, Y.-M.: Colorimetric sensing of Cu(II): Cu(II) induced deprotonation of an amide responsible for color changes. Dalton Trans. 39, 4363–4368 (2010)
Fabbrizzi, L., Licchelli, M., Pallavicini, P., Perotti, A., Sacchi, D.: An anthracene-based fluorescent sensor for transition metal ions. Angew. Chem. 33, 19 (1994)
Huang, J., Xu, Y., Qian, X.: A red-shift colorimetric and fluorescent sensor for Cu2+ in aqueous solution: unsymmetrical 4,5-diaminonaphthalimide with N–H deprotonation induced by metal ions. Org. Biomol. Chem. 7, 1299–1303 (2009)
Jiang, J., Jiang, H., Tang, X., Yang, L., Dou, W., Liu, W., Fang, R., Liu, W.: An efficient sensor for Zn2+ and Cu2+ based on different binding modes. Dalton Trans. 40, 6367–6370 (2011)
Meyer, M., Fre′mond, L., Espinosa, E., Guilard, R., Ou, Z., Kadish, K.M.: Synthesis, characterization, and X-ray crystal structures of cyclam derivatives. 5. Copper(II) binding studies of a pyridine-strapped 5,12-dioxocyclam-based macrobicycle. Inorg. Chem. 43, 18 (2004)
Acknowledgments
Preeti Goel and Richa Shukla thank UGC and CSIR, India resepctively for research fellowship. Financial assistance from DST, MFPI, MOEF and MoRD is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chawla, H.M., Goel, P., Shukla, R. et al. New lower rim looped calix[4]arene for ratiometric and chromogenic recognition of Cu2+ . J Incl Phenom Macrocycl Chem 80, 201–207 (2014). https://doi.org/10.1007/s10847-013-0376-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-013-0376-0