Abstract
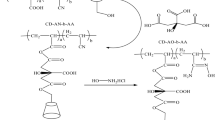
Batch adsorption experiments were carried out for the removal of ponceau 4R (P4R, C.I. 16255) from aqueous solutions using a novel polyamidoamine–cyclodextrin crosslinked copolymer (PAMAM-CD). The influence of several operating variables, such as contact time, initial concentration, pH, ionic strength, was investigated. Results showed that PAMAM-CD exhibited very high adsorption capacity toward P4R. The adsorption capacity of P4R was even up to 254.3 mg/g when the initial concentration of P4R solution was 340 mg/L at 288 K. The maximum adsorption capacity occured at below pH 5. The adsorption rate was fast and over 52% of the equilibrium adsorption value occurred in the first 15 min at 308 K. Adorption kinetics followed the Ho and McKay equation. Intraparticle diffusion was involved in the adsorption process but it is not the only rate-controlling step. Equilibrium isotherm data were precisely fitted by the Langmuir model. The negative values of Gibbs free energy change indicated the spontaneous nature of adsorption. The PAMAM-CD was easily recovered by 2 M HCl as washing solvent and it could be used as a promising alternative adsorbent.










Similar content being viewed by others
References
Tanaka, T.: Reproductive and neurobehavioural toxicity study of ponceau 4R administered to mice in the diet. Food Chem. Toxicol. 44, 1651–1658 (2006)
Oliveira, D.F.M., Batista, P.S., Muller Jr., P.S., Velani, V., França, M.D., Souza, D.R., Machado, A.E.H.: Evaluating the effectiveness of photocatalysts based on titanium dioxide in the degradation of the dye ponceau 4R. Dyes Pigm. 92, 563–572 (2011)
Sadik, W.A., Nashed, A.W., El-Demerdash, A.G.M.: Photodecolourization of ponceau 4R by heterogeneous photocatalysis. J. Photochem. Photobiol. A Chem. 189, 135–140 (2007)
Chagas, E.P., Durrant, L.R.: Decolorization of azo dyes by Phanerochaete chrysosporium and Pleurotus sajorcaju. Enzyme Microb. Technol. 29, 473–477 (2001)
Huang, Y.F., Huang, Y.H., Gemeay, A.H., Habib, A.F.M., El-Din, M.A.B.: Kinetics and mechanism of the uncatalyzed and Ag(I)-catalyzed oxidative decolorization of Sunset Yellow and ponceau 4R with peroxydisulphate. Dyes Pigm 74, 458–463 (2007)
Salem, M.A., Abdel-Halim, S.T., El-Sawy, A.E.H.M., Zaki, A.B.: Kinetics of degradation of allura red, ponceau 4R and carmosine dyes with potassium ferrioxalate complex in the presence of H2O2. Chemosphere 76, 1088–1093 (2009)
Dragan, S., Cristea, M., Airinei, A., Poinescu, I., Luca, C.: Sorption of aromatic compounds on macroporous anion exchangers based on polyacrylamide: relation between structure and sorption behavior. J. Appl. Polym. Sci. 55, 421–430 (1995)
Crini, G.: Non-conventional low-cost adsorbents for dye removal: a review. Bioresour. Technol. 97, 1061–1085 (2006)
Mittal, A.: Adsorption kinetics of removal of a toxic dye, malachite green, from wastewater by using hen feathers. J. Hazard. Mater. 113, 196–202 (2006)
Rahchamani, J., Mousavi, H.Z., Behzad, M.: Adsorption of methyl violet from aqueous solution by polyacrylamide as an adsorbent: isotherm and kinetic studies. Desalination 267, 256–260 (2011)
Likozar, B., Senica, D., Pavko, A.: Equilibrium and kinetics of vancomycin adsorption on polymeric adsorbent. AIChE J. (2011). doi:10.1002/aic.12559
Pan, J., Zou, X., Wang, X., Guan, W., Li, C., Yan, Y., Wu, X.: Adsorption removal of 2,4-dichlorophenol and 2,6-dichlorophenol from aqueous solution by β-cyclodextrin/attapulgite composites: equilibrium, kinetics and thermodynamics. Chem. Eng. J. 166, 40–48 (2011)
Del Valle, E.M.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Crini, G., Peindy, H.N., Gimbert, F., Robert, C.: Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep. Sci. Technol. 53, 97–110 (2007)
Li, N., Mei, Z., Ding, S.G.: 2,4-Dichlorophenol sorption on cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem. 68, 123–129 (2010)
Carmen, G., Miguel, S., José, R.I., Itziar, V., Carmen, M., Cristina, M.O., Arantza, Z.: Sorption of pindolol and related compounds by a β-cyclodextrin polymer: isosteric heat of sorption. Carbohydr. Polym. 71, 140–146 (2008)
Klajnert, B., Epand, R.M.: PAMAM dendrimers and model membranes: differential scanning calorimetry studies. Int. J. Pharm. 305, 154–166 (2005)
Rether, A., Schuster, M.: Selective separation and recovery of heavy metal ions using water-soluble N-benzoylthiourea modified PAMAM polymers. React. Funct. Polym. 57, 13–21 (2003)
Huang, Y.; Kang, Q.: Synthesis of conjugates of β-cyclodextrin with polyamidoamine dendrimers and their molecular inclusion interaction with levofloxacin lactate. J. Incl. Phenom. Macrocycl. Chem. (2011). doi:10.1007/s10847-011-9938-1
Li, N., Wei, X.Y., Mei, Z., Xiong, X.L., Chen, S.M., Ye, M., Ding, S.G.: Synthesis and characterization of a novel polyamidoamine–cyclodextrin crosslinked copolymer. Carbohydr. Res. 346, 1721–1727 (2011)
Yoe, J.H., Jones, A.L.: Colorimetric determination of iron with disodium-1,2-dihydroxybenzene-3,5-disulfonate. Ind. Eng. Chem. Anal. Ed. 16, 111–115 (1944)
Ho, Y.S., McKay, G.: Pseudo-second order model for sorption processes. Process Biochem. 34, 451–465 (1999)
Wu, Z., Joo, H., Lee, K.: Kinetics and thermodynamics of the organic dye adsorption on the mesoporous hybrid xerogel. Chem. Eng. J. 112, 227–236 (2005)
Kalavathy, M.H., Karthikeyan, T., Rajgopal, S., Mira, L.R.: Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4-activated rubber wood sawdust. J. Colloid Interface Sci. 292, 354–362 (2005)
Weber, W.J., Morris, J.: Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Proc. Am. Soc. Civ. Eng. SA2, 31–59 (1963)
Hameed, B.H., Rahman, A.A.: Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 160, 576–581 (2008)
Gong, R.M., Zhu, S.X., Zhang, D.M., Chen, J., Ni, S.J., Guan, R.: Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: kinetic and thermodynamic profile. Desalination 230, 220–228 (2008)
Hameed, B.H., El-Khaiary, M.I.: Kinetics and equilibrium studies of malachite green adsorption on rice straw-derived char. J. Hazard. Mater. 153, 701–708 (2008)
Temkin, M.J., Pyzhev, V.: Recent modifications to Langmiur isotherms. Acta Physiochim. USSR 12, 217–225 (1940)
Rand, B.: On the empirical nature of the Dubinin-Radushkevich equation of adsorption. J. Colloid Interface Sci. 56, 337–346 (1976)
Freundlich, H.M.F.: Over the adsorption in solution. J. Phys. Chem. 57, 385–470 (1906)
Langmuir, I.: The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 38, 2221–2295 (1916)
Ozmen, E.Y., Yilmaz, M.: Use of β-cyclodextrin and starch based polymers for sorption of congo red from aqueous solutions. J. Hazard. Mater. 148, 303–310 (2007)
Wawrzkiewicz, M., Hubicki, Z.: Equilibrium and kinetic studies on the adsorption of acidic dye by the gel anion exchanger. J. Hazard. Mater. 172, 868–874 (2009)
Hall, K.R., Eagleton, L.C., Acrivos, A., Vermeulen, T.: Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 5, 212–223 (1966)
Weber, T.W., Chakravorti, R.K.: Pore and solid diffusion models for fixed bed adsorbents. AIChE J. 20, 228–238 (1974)
Hosangadi, B., Palekar, S.: Preferential inclusion of food colours via formation of their inclusion complexes with/β-cyclodextrin (β-CD). J. Incl. Phenom. Mol. Recognit. Chem. 7, 321–325 (1989)
Taguchi, T., Hirayama, S., Okamoto, M.: New spectroscopic evidence for molecular aggregates of rhodamine 6G in aqueous solution at high pressure. Chem. Phys. Lett. 231, 561–568 (1994)
Chatterjee, S., Chatterjee, S., Chatterjee, B.P., Das, A.R., Guha, A.K.: Adsorption of a model anionic dye, eosin Y, from aqueous solution by chitosan hydrobeads. J. Colloid Interface Sci. 288, 30–35 (2005)
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 20907076), the Key Research Project for Discipline Construction of Chongqing Technology & Business University (No. 1152006), and the Program for Innovation Team Building in University (Chongqing Municipal Education Commission of China. No. KJTD201020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, N., Lei, XM. Adsorption of ponceau 4R from aqueous solutions by polyamidoamine–cyclodextrin crosslinked copolymer. J Incl Phenom Macrocycl Chem 74, 167–176 (2012). https://doi.org/10.1007/s10847-011-0096-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-0096-2