Abstract
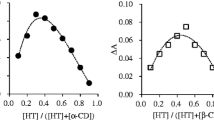
A series of cyclodextrin/scutellarin inclusion complexes were prepared from α-cyclodextrin, β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin with scutellarin (SCU), and their inclusion complexation behaviors, such as stoichiometry, complex stability constants and inclusion mode, were investigated by means of UV/Vis spectroscopy, 1H NMR and 2D NMR. The results showed that the SCU could be efficiently encapsulated in the cyclodextrin cavity in aqueous solution to produce complexes that were more soluble than free SCU. The enhanced binding ability of cyclodextrins towards SCU was discussed from the viewpoint of the size/shape-fit and multiple recognition mechanism between host and guest.









Similar content being viewed by others
References
Zhang, W.D., Chen, W.S., Wang, Y.H., Yang, G.J., Kong, D.Y., Li, H.T.: Studies on the flavone glycosides from the extract of Erigeron breviscapus. Chin. Traditional Herb. Drugs. 31, 565–568 (2000)
Zhang, J., Li, X.S., Zhang, W.D.: Recent progress on pharmacologic activity and chemical components of breviscapine. J. Pharm. Pract. 20, 103–107 (2002)
Deng, T.F.: Clinical application of breviscapine injection in Chinese patients. China Pharm. 11, 728–730 (2002)
Si, S.L., Xu, L.Y.: Clinical application of breviscapine formulations. Chin. J. Clin. Pharm. 13, 408–410 (2004)
Ju, W.Z., Chun, J.H., Tan, R.X., Xiong, N.N.: Study on metabolites of scutellarin in gastrointestinal tract by UPLC-MS/MS method. Chin. J. Clin. Pharmacol. Ther. 11, 292–295 (2006)
Jiang, X.H., Li, S.H., Lan, K., Yang, J.Y., Zhou, J.: Study on the pharmacokinetics of scutellarin in dogs. Acta. Pharm. Sinica. 38, 371–373 (2003)
Liu, Y.M., Lin, A.H., Chen, H., Zeng, F.D.: Study on pharmacokinetics of scutellarin in rabbits. Acta. Pharm. Sinica. 38, 775–778 (2003)
Ge, Q.-H., Zhou, Z., Zhi, X.J., Ma, L.L.: Pharmacokinetics and absolute bioavailability of breviscapine in Beagle dogs. Chin. J. Pharm. 34, 618–620 (2003)
Hong, H., Liu, G.Q.: Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 74, 2959–2973 (2004)
David, G., Yian, H.L., Eng, S.O.: Inhibitory effects of a chemically standardized extract from scutellaria barbata in human colon cancer cell lines, LoVo. J. Agric. Food Chem. 53, 8197–8204 (2005)
Shuai, J., Dong, W.W.: Experimental research of PKC inhibitor. Erigeron breviscapus on the ischemic/reperfusional brain injury. Chin. Pharmacol. Bull. 14, 75–77 (1998)
Szejtli, J.: Cyclodextrin Technology, pp. 450–455. Kluwer Academic Publisher, Dordrecht (1988)
Uekama, K., Hirayama, F., Irie, T.: Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045–2076 (1998)
Loftsson, T., Järvinen, T.: Cyclodextrins in ophthalmic drug delivery. Adv. Drug Deliv. Rev. 36, 59–79 (1999)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85, 1017–1025 (1996)
Inoue, Y., Yamamoto, K., Wada, T., Everitt, S., Gao, X.M., Hou, Z.J., Tong, L.H., Jiang, S.K., Wu, H.M.: Inclusion complexation of (cyclo)alkanes and (cyclo)alkanols with 6-O-modified cyclodextrins. J. Chem. Soc. Perkin Trans. 2, 1807–1816 (1998)
Liu, Y., Li, B., Wada, T., Inoue, Y.: Novel O-Phenylenediseleno bridged Bis(β-cyclodextrin)s complexes with Platinum(IV) and Palladium(II) Ions. Supramol. Chem. 10, 279–285 (1999)
Reinhardt, R., Richter, M., Mager, P.P.: Investigation of the conformational behaviour of permethylated cyclodextrins by molecular modelling. Carbohydrate Res. 291, 1–9 (1996)
Kano, K., Nishiyabu, R., Asada, T., Kuroda, Y.: Static and dynamic behavior of 2:1 inclusion complexes of cyclodextrins and charged porphyrins in aqueous organic media. J. Am. Chem. Soc. 124, 9937–9944 (2002)
Yi, Z.-P., Chen, H.-L., Huang, Z.-Z., Huang, Q., Yu, J.-S.: Contributions of weak interactions to the inclusion complexation of 3-hydroxynaphthalene-2-carboxylic acid and its analogues with cyclodextrins. J. Chem. Soc. Perkin Trans. 2, 121–127 (2000)
Linares, M., de Bertorello, M.M., Longhi, M.: Solubilization of naphthoquinones by complexation with hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 159, 13–18 (1997)
Liu, Y., Chen, C.-S., Chen, Y., Lin, J.: Inclusion complexes of azadirachtin with native and methylated cyclodextrins: solubilization and binding ability. Bioorg. Med. Chem. 13, 4037–4042 (2005)
de Araújo, M.V.G.: Sulfadiazine/hydroxypropyl-β-cyclodextrin host–guest system: characterization, phase-solubility and molecular modeling. Bioorg. Med. Chem. 16, 5788–5794 (2008)
Correia, I., Bezzenine, N., Ronzani, N., Platzer, N., Beloeil, J.-C., Doan, B.-T.: Study of inclusion complexes of acridine with β- and (2,6-di-O-methyl)-β-cyclodextrin by use of solubility diagrams and NMR spectroscopy. J. Phys. Org. Chem. 15, 647–659 (2002)
Montassier, P., Duchêne, D., Poelman, M.C.: Inclusion complexes of tretinoin with cyclodextrins. Int. J. Pharm. 153, 199–209 (1997)
Acknowledgments
This work was supported by the Opening Foundation of State Key Laboratory of Elemento-Organic Chemistry of Nankai University (0607 and 0704), which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, B., Yang, LJ., Lin, J. et al. Binding behaviors of scutellarin with α-, β-, γ-cyclodextrins and their derivatives. J Incl Phenom Macrocycl Chem 64, 149–155 (2009). https://doi.org/10.1007/s10847-009-9547-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9547-4