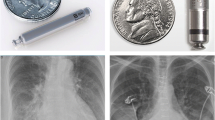
Abstract
Background
Roughly one in six patients receiving conventional transvenous pacemaker systems experience significant complications within 1 year of implant, mainly due to the transvenous lead and subcutaneous pocket. A new helix-fixation single-chamber ventricular leadless pacemaker (LP) system capable of pre-deployment exploratory electrical mapping is commercially available. Such an LP may mitigate complications while streamlining the implantation. In this study, the initial real-world implant experience of the helix-fixation LP was evaluated following its commercial release.
Methods
In patients indicated for single-chamber right ventricular pacing, helix-fixation Aveir VR LPs (Abbott, Abbott Park, IL) were implanted using the dedicated loading tool, introducer, and delivery catheter. Implant procedural characteristics, electrical parameters, and any 30-day procedure-related adverse events of consecutive implant attempts were retrospectively evaluated.
Results
A total of 167 patients with Class I indication for permanent pacing received implants in four North American centers (57% male, 70 years old). Pre-fixation electrical mapping of potential sites allowed repositioning to be avoided in 95.7% of patients. Median [interquartile range] LP procedure and fluoroscopy durations were 25.5 min [20.0, 35.0] and 5.7 min [4.0, 9.2], respectively. Pacing capture threshold, sensed R-wave amplitude, and impedance were 0.8 V [0.5, 1.3], 9.0 mV [6.0, 12.0], and 705 Ω [550, 910], respectively. Implantation was successful in 98.8% of patients, with 98.2% free from acute adverse events.
Conclusions
The initial, real-world experience of the helix-fixation ventricular leadless pacemaker demonstrated safe and efficient implantation with minimal repositioning, viable electrical metrics, and limited acute complications.
Graphical abstract
Helix-fixation single-chamber ventricular leadless pacemakers were implanted in 167 patients, with a median procedure time of 25.5 minutes, 98.8% implant success, and 98.2% freedom from acute adverse events.







Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Cantillon DJ, et al. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC: Clinical Electrophysiology. 2017;3(11):1296–305.
Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35(18):1186–94.
Udo EO, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;9(5):728–35.
Tobin K, Stewart J, Westveer D, Frumin H. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol. 2000;85(6):774–6.
Sattar Y, et al. Complications of leadless vs conventional (lead) artificial pacemakers – a retrospective review. J Community Hosp Intern Med Perspect. 2020;10(4):328–33.
Tjong FVY, Reddy VY. Permanent leadless cardiac pacemaker therapy: a comprehensive review. Circulation. 2017;135(15):1458–70.
Reddy VY, et al. Primary results on safety and efficacy from the LEADLESS II-phase 2 worldwide clinical trial. JACC Clin Electrophysiol. 2022;8(1):115–7.
Roberts PR, et al. A leadless pacemaker in the real-world setting: the micra transcatheter pacing system post-approval registry. Heart Rhythm. 2017;14(9):1375–9.
Reddy VY, et al. 1-year outcomes of a leadless ventricular pacemaker: the LEADLESS II (phase 2) trial. JACC Clin Electrophysiol. 2023;9(7 Pt 2):1187–9.
Funding
No funds, grants, or other support was received. Data analysis and manuscript preparation supported by Abbott.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving human participants and/or animals
This retrospective study was performed according to the principles outlined in the Declaration of Helsinki and the Good Clinical Practice guidelines of the European Commission and was approved by the local ethics committee.
Informed consent
All patients provided written informed consent.
Conflict of interest
DGN has served on the advisory board, served as a consultant, or received honoraria, research grants, or support from Medtronic Inc., Boston Scientific Corporation, Abbott, Biosense Webster, and Adagio Medical. DVE has served as a consultant and received honoraria and research grants from Abbott; and unrelated to this manuscript, serves as a consultant for Boston Scientific, Medtronic, and GE Healthcare and has equity in Clarius, CorVista eMurmur, HelpWear, and ProtonIntel. VYR has served as a consultant to Abbott; and unrelated to this manuscript, he serves as a consultant for and has equity in Ablacon, Acutus Medical, Affera-Medtronic, Apama Medical-Boston Scientific, Anumana, APN Health, Aquaheart, Atacor, Autonomix, Axon Therapies, Backbeat, BioSig, CardiaCare, CardioNXT/AFTx, Circa Scientific, CoRISMA, Corvia Medical, Dinova-Hangzhou DiNovA EP Technology, East End Medical, EPD-Philips, EP Frontiers, Epix Therapeutics-Medtronic, EpiEP, Eximo, Farapulse-Boston Scientific, Field Medical, Focused Therapeutics, HRT, Intershunt, Javelin, Kardium, Keystone Heart, LuxMed, Medlumics, Middlepeak, Neutrace, Nuvera-Biosense Webster, Oracle Health, Restore Medical, Sirona Medical, SoundCath, Valcare; unrelated to this work, has served as a consultant for AtriAN, Biosense-Webster, BioTel Heart, Biotronik, Boston Scientific, Cairdac, Cardiofocus, Cardionomic, CoreMap, Fire1, Gore & Associates, Impulse Dynamics, Medtronic, Novartis, Philips, Pulse Biosciences; and has equity in DRS Vascular, Manual Surgical Sciences, Newpace, Nyra Medical, Surecor, and Vizaramed. NB and DL are employees of Abbott. ZE received consulting honoraria from AtriCure. MI received honoraria from Boston Scientific and Biosense Webster. CH served as a consultant or received honoraria, research grants, or support from Abbott and Biotronik. NB and DL are employees of Abbott.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nair, D.G., Exner, D.V., Reddy, V.Y. et al. Early real-world implant experience with a helix-fixation ventricular leadless pacemaker. J Interv Card Electrophysiol (2024). https://doi.org/10.1007/s10840-024-01791-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10840-024-01791-1