Abstract
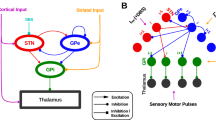
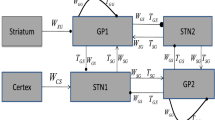
Parkinson’s disease (PD) and animal models of PD feature enhanced oscillations in several frequency bands in the basal ganglia (BG). Past research has emphasized the enhancement of 13-30 Hz beta oscillations. Recently, however, oscillations in the delta band (0.5-4 Hz) have been identified as a robust predictor of dopamine loss and motor dysfunction in several BG regions in mouse models of PD. In particular, delta oscillations in the substantia nigra pars reticulata (SNr) were shown to lead oscillations in motor cortex (M1) and persist under M1 lesion, but it is not clear where these oscillations are initially generated. In this paper, we use a computational model to study how delta oscillations may arise in the SNr due to projections from the globus pallidus externa (GPe). We propose a network architecture that incorporates inhibition in SNr from oscillating GPe neurons and other SNr neurons. In our simulations, this configuration yields firing patterns in model SNr neurons that match those measured in vivo. In particular, we see the spontaneous emergence of near-antiphase active-predicting and inactive-predicting neural populations in the SNr, which persist under the inclusion of STN inputs based on experimental recordings. These results demonstrate how delta oscillations can propagate through BG nuclei despite imperfect oscillatory synchrony in the source site, narrowing down potential targets for the source of delta oscillations in PD models and giving new insight into the dynamics of SNr oscillations.







Similar content being viewed by others
Data availability
No new data was utlized in this work.
Code availability
Computational model and analysis code is currently available at the following site: https://github.com/jparker25/Whalen_et_al_2021
References
Abbott, L. F., Varela, J., Sen, K., & Nelson, S. (1997). Synaptic depression and cortical gain control. Science, 275(5297), 221–224.
Abdi, A., Mallet, N., Mohamed, F. Y., Sharott, A., Dodson, P. D., Nakamura, K. C., Suri, S., Avery, S. V., Larvin, J. T., Garas, F. N., et al. (2015). Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. Journal of Neuroscience, 35(17), 6667–6688.
Aristieta, A., Ruiz-Ortega, J., Miguelez, C., Morera-Herreras, T., & Ugedo, L. (2016). Chronic l-dopa administration increases the firing rate but does not reverse enhanced slow frequency oscillatory activity and synchronization in substantia nigra pars reticulata neurons from 6-hydroxydopamine-lesioned rats. Neurobiology of Disease, 89, 88–100.
Boraud, T., Brown, P., Goldberg, J. A., Graybiel, A. M., Magill, P. J. (2005). Oscillations in the basal ganglia: the good, the bad, and the unexpected. In: The basal ganglia VIII, Springer, pp 1–24
Brown, P., Oliviero, A., Mazzone, P., Insola, A., Tonali, P., & Di Lazzaro, V. (2001). Dopamine dependency of oscillations between subthalamic nucleus and pallidum in parkinson’s disease. Journal of Neuroscience, 21(3), 1033–1038.
Cancedda, L., & Poo, M. M. (2009). Synapse formation and elimination: competition and the role of activity. In: Binder M, Hirokawa N, Windhorst U (eds) Encyclopedia of Neuroscience, Springer, Berlin, Heidelberg, pp 3932–3938
Cassidy, M., Mazzone, P., Oliviero, A., Insola, A., Tonali, P., Lazzaro, V. D., & Brown, P. (2002). Movement-related changes in synchronization in the human basal ganglia. Brain, 125(6), 1235–1246.
Connelly, W. M., Schulz, J. M., Lees, G., & Reynolds, J. N. (2010). Differential short-term plasticity at convergent inhibitory synapses to the substantia nigra pars reticulata. Journal of Neuroscience, 30(44), 14854–14861.
Corbit, V. L., Whalen, T. C., Zitelli, K. T., Crilly, S. Y., Rubin, J. E., & Gittis, A. H. (2016). Pallidostriatal projections promote \(\beta\) oscillations in a dopamine-depleted biophysical network model. Journal of Neuroscience, 36(20), 5556–5571.
DeLong, M., & Wichmann, T. (2010). Changing views of basal ganglia circuits and circuit disorders. Clinical EEG and neuroscience, 41(2), 61–67.
Du, G., Zhuang, P., Hallett, M., Zhang, Y. Q., Li, J. Y., & Li, Y. J. (2018). Properties of oscillatory neuronal activity in the basal ganglia and thalamus in patients with parkinson’s disease. Translational neurodegeneration, 7(1), 1–13.
Fino, E., Glowinski, J., & Venance, L. (2005). Bidirectional activity-dependent plasticity at corticostriatal synapses. Journal of Neuroscience, 25(49), 11279–11287.
Foster, N. N., Korobkova, L., Garcia, L., Gao, L., Becerra, M., Sherafat, Y., Peng, B., Li, X., Choi, J. H., Gou, L., et al. (2020). The mouse cortico-basal ganglia-thalamic network. bioRxiv
Freeze, B. S., Kravitz, A. V., Hammack, N., Berke, J. D., & Kreitzer, A. C. (2013). Control of basal ganglia output by direct and indirect pathway projection neurons. Journal of Neuroscience, 33(47), 18531–18539.
Gerstner, W., Kistler, W. M., Naud, R., Paninski, L. (2014). Neuronal dynamics: From single neurons to networks and models of cognition. Cambridge University Press
Halje, P., Brys, I., Mariman, J. J., da Cunha, C., Fuentes, R., & Petersson, P. (2019). Oscillations in cortico-basal ganglia circuits: Implications for parkinson’s disease and other neurologic and psychiatric conditions. Journal of Neurophysiology, 122(1), 203–231.
Hammond, C., Bergman, H., & Brown, P. (2007). Pathological synchronization in parkinson’s disease: networks, models and treatments. Trends in Neurosciences, 30(7), 357–364.
Heimer, G., Rivlin, M., Israel, Z., Bergman, H. (2006). Synchronizing activity of basal ganglia and pathophysiology of parkinson’s disease. Parkinson’s Disease and Related Disorders, pp 17–20
Higgs, M. H., & Wilson, C. J. (2016). Unitary synaptic connections among substantia nigra pars reticulata neurons. Journal of Neurophysiology, 115(6), 2814–2829.
Holt, G. R., Softky, W. R., Koch, C., & Douglas, R. J. (1996). Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. Journal of Neurophysiology, 75(5), 1806–1814.
Hurtado, J. M., Gray, C. M., Tamas, L. B., & Sigvardt, K. A. (1999). Dynamics of tremor-related oscillations in the human globus pallidus: a single case study. Proceedings of the National Academy of Sciences, 96(4), 1674–1679.
Hurtado, J. M., Rubchinsky, L. L., Sigvardt, K. A., Wheelock, V. L., & Pappas, C. T. (2005). Temporal evolution of oscillations and synchrony in gpi/muscle pairs in parkinson’s disease. Journal of Neurophysiology, 93(3), 1569–1584.
Jenkinson, N., & Brown, P. (2011). New insights into the relationship between dopamine, beta oscillations and motor function. Trends in Neurosciences, 34(12), 611–618.
Kita, H., & Kitai, S. (1987). Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the pha-l method. Journal of Comparative Neurology, 260(3), 435–452.
Levy, R., Ashby, P., Hutchison, W. D., Lang, A. E., Lozano, A. M., & Dostrovsky, J. O. (2002). Dependence of subthalamic nucleus oscillations on movement and dopamine in parkinson’s disease. Brain, 125(6), 1196–1209.
Lo, Y. J., & Poo, M. M. (1991). Activity-dependent synaptic competition in vitro: heterosynaptic suppression of developing synapses. Science, 254(5034), 1019–1022.
Mallet, N., Pogosyan, A., Márton, L. F., Bolam, J. P., Brown, P., & Magill, P. J. (2008). Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. Journal of Neuroscience, 28(52), 14245–14258.
Mallet, N., Micklem, B. R., Henny, P., Brown, M. T., Williams, C., Bolam, J. P., Nakamura, K. C., & Magill, P. J. (2012). Dichotomous organization of the external globus pallidus. Neuron, 74(6), 1075–1086.
McCairn, K. W., & Turner, R. S. (2009). Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. Journal of Neurophysiology, 101(4), 1941–1960.
Nakano, K. (2000). Neural circuits and topographic organization of the basal ganglia and related regions. Brain and Development, 22, 5–16.
Parr-Brownlie, L. C., Poloskey, S. L., Bergstrom, D. A., & Walters, J. R. (2009). Parafascicular thalamic nucleus activity in a rat model of parkinson’s disease. Experimental Neurology, 217(2), 269–281.
Phillips, R. S., Rosner, I., Gittis, A. H., & Rubin, J. E. (2020). The effects of chloride dynamics on substantia nigra pars reticulata responses to pallidal and striatal inputs. Elife, 9, e55592.
Raz, A., Vaadia, E., & Bergman, H. (2000). Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine vervet model of parkinsonism. Journal of Neuroscience, 20(22), 8559–8571.
Rubin, J. E. (2017). Computational models of basal ganglia dysfunction: the dynamics is in the details. Current Opinion in Neurobiology, 46, 127–135.
Simmons, D. V., Higgs, M. H., Lebby, S., & Wilson, C. J. (2020). Indirect pathway control of firing rate and pattern in the substantia nigra pars reticulata. Journal of Neurophysiology, 123(2), 800–814.
Smith, Y., & Bolam, J. P. (1989). Neurons of the substantia nigra reticulata receive a dense gaba-containing input from the globus pallidus in the rat. Brain Research, 493(1), 160–167.
Steigerwald, F., Potter, M., Herzog, J., Pinsker, M., Kopper, F., Mehdorn, H., Deuschl, G., & Volkmann, J. (2008). Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. Journal of Neurophysiology, 100(5), 2515–2524.
Steriade, M., McCormick, D. A., & Sejnowski, T. J. (1993). Thalamocortical oscillations in the sleeping and aroused brain. Science, 262(5134), 679–685.
Terman, D., Rubin, J. E., Yew, A., & Wilson, C. (2002). Activity patterns in a model for the subthalamopallidal network of the basal ganglia. Journal of Neuroscience, 22(7), 2963–2976.
Thoenen, H. (2000). Neurotrophins and activity-dependent plasticity. Progress in Brain Research, 128, 183–191.
Tseng, K. Y., Kasanetz, F., Kargieman, L., Riquelme, L. A., & Murer, M. G. (2001). Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. Journal of Neuroscience, 21(16), 6430–6439.
Turrigiano, G. G. (2008). The self-tuning neuron: synaptic scaling of excitatory synapses. Cell, 135(3), 422–435.
Walters, J. R., Hu, D., Itoga, C. A., Parr-Brownlie, L. C., & Bergstrom, D. A. (2007). Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience, 144(2), 762–776.
Weinberger, M., Mahant, N., Hutchison, W. D., Lozano, A. M., Moro, E., Hodaie, M., Lang, A. E., & Dostrovsky, J. O. (2006). Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in parkinson’s disease. Journal of Neurophysiology, 96(6), 3248–3256.
Whalen, T. C., Willard, A. M., Rubin, J. E., & Gittis, A. H. (2020). Delta oscillations are a robust biomarker of dopamine depletion severity and motor dysfunction in awake mice. Journal of Neurophysiology, 124(2), 312–329.
Willard, A. M., Isett, B. R., Whalen, T. C., Mastro, K. J., Ki, C. S., Mao, X., & Gittis, A. H. (2019). State transitions in the substantia nigra reticulata predict the onset of motor deficits in models of progressive dopamine depletion in mice. Elife, 8, e42746.
Xia, X. M., Fakler, B., Rivard, A., Wayman, G., Johnson-Pais, T., Keen, J., Ishii, T., Hirschberg, B., Bond, C., Lutsenko, S., et al. (1998). Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature, 395(6701), 503.
Yelnik, A. P., Lebreton, F. O., Bonan, I. V., Colle, F. M., Meurin, F. A., Guichard, J. P., & Vicaut, E. (2002). Perception of verticality after recent cerebral hemispheric stroke. Stroke, 33(9), 2247–2253.
Zhou, F. W., Matta, S. G., & Zhou, F. M. (2008). Constitutively active trpc3 channels regulate basal ganglia output neurons. Journal of Neuroscience, 28(2), 473–482.
Zhou, F. W., Jin, Y., Matta, S. G., Xu, M., & Zhou, F. M. (2009). An ultra-short dopamine pathway regulates basal ganglia output. Journal of Neuroscience, 29(33), 10424–10435.
Zhuang, P., Hallett, M., Meng, D., Zhang, Y., & Li, Y. (2019). Characteristics of oscillatory activity in the globus pallidus internus in patients with parkinson’s disease (p1.8-028). Neurology, 92(15 Supplement).
Acknowledgements
We thank Ryan Phillips for providing the C++ code upon which our biophysical model was based and for helpful discussions regarding our changes and tuning of the finished model. We also thank Charles J. Wilson for suggesting the comparison with the integrate-and-fire model.
Funding
This work was partially supported by NSF award DMS 1951095 (JER) and by NIH awards R01DA053014 (JER), R01NS125914 (JER/AHG), R01NS101016 (AHG), R01NS104835 (AHG), and F31NS101821 (TCW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Action Editor: Charles Wilson
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Whalen, T.C., Parker, J.E., Gittis, A.H. et al. Transmission of delta band (0.5-4 Hz) oscillations from the globus pallidus to the substantia nigra pars reticulata in dopamine depletion. J Comput Neurosci 51, 361–380 (2023). https://doi.org/10.1007/s10827-023-00853-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-023-00853-z