Abstract
Purpose
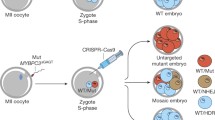
Unpredictable genetic modifications and chromosomal aberrations following CRISPR/Cas9 administration hamper the efficacy of germline editing. Repair events triggered by double-strand DNA breaks (DSBs) besides non-homologous end joining and repair template-driven homology-directed repair have been insufficiently investigated in mouse. In this work, we are the first to investigate the precise repair mechanisms triggered by parental-specific DSB induction in mouse for paternal mutational correction in the context of an infertility-related mutation.
Methods
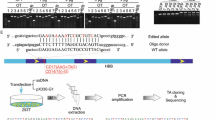
We aimed to correct a paternal 22-nucleotide deletion in Plcz1, associated with lack of fertilisation in vitro, by administrating CRISPR/Cas9 components during intracytoplasmic injection of Plcz1-null sperm in wild-type oocytes combined with assisted oocyte activation. Through targeted next-generation sequencing, 77 injected embryos and 26 blastomeres from seven injected embryos were investigated. In addition, low-pass whole genome sequencing was successfully performed on 17 injected embryo samples.
Results
Repair mechanisms induced by two different CRISPR/Cas9 guide RNA (gRNA) designs were investigated. In 13.73% (7/51; gRNA 1) and 19.05% (4/21; gRNA 2) of the targeted embryos, only the wild-type allele was observed, of which the majority (85.71%; 6/7) showed integrity of the targeted chromosome. Remarkably, for both designs, only in one of these embryos (1/7; gRNA 1 and 1/4; gRNA2) could repair template use be detected. This suggests that alternative repair events have occurred. Next, various genetic events within the same embryo were detected after single-cell analysis of four embryos.
Conclusion
Our results suggest the occurrence of mosaicism and complex repair events after CRISPR/Cas9 DSB induction where chromosomal integrity is predominantly contained.




Similar content being viewed by others
Data availability
Data is available upon reasonable request.
Code availability
Not applicable.
References
Plaza Reyes A. and Lanner F., Towards a CRISPR view of early human development: applications, limitations and ethical concerns of genome editing in human embryos, Development (Cambridge, England), 2017, https://doi.org/10.1242/dev.139683.
Gaj T, Gersbach CA, Barbas CF, 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013.https://doi.org/10.1016/j.tibtech.2013.04.004
Sansbury BM, Hewes AM, Kmiec EB. Understanding the diversity of genetic outcomes from CRISPR-Cas generated homology-directed repair. Commun Biol. 2019. https://doi.org/10.1038/s42003-019-0705-y.
Jiang F, Doudna JA. CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys. 2017. https://doi.org/10.1146/annurev-biophys-062215-010822.
Tang L, et al. CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol Genet Genomics : MGG. 2017. https://doi.org/10.1007/s00438-017-1299-z.
Liang D, et al. Limitations of gene editing assessments in human preimplantation embryos. Nat Commun. 2023. https://doi.org/10.1038/s41467-023-36820-6.
Zuccaro MV, et al. Allele-specific chromosome removal after Cas9 cleavage in human embryos. Cell. 2020. https://doi.org/10.1016/j.cell.2020.10.025.
Ma H, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017. https://doi.org/10.1038/nature23305.
Bekaert B, et al. Retained chromosomal integrity following CRISPR-Cas9-based mutational correction in human embryos. Mol Ther : J Am Soc Gene Ther. 2023. https://doi.org/10.1016/j.ymthe.2023.06.013.
Wu Y, et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell. 2013. https://doi.org/10.1016/j.stem.2013.10.016.
Mianne J, et al. Correction of the auditory phenotype in C57BL/6N mice via CRISPR/Cas9-mediated homology directed repair. Genome medicine. 2016. https://doi.org/10.1186/s13073-016-0273-4.
Huai C, et al. CRISPR/Cas9-mediated somatic and germline gene correction to restore hemostasis in hemophilia B mice. Hum Genet. 2017. https://doi.org/10.1007/s00439-017-1801-z.
Wu WH, et al. CRISPR repair reveals causative mutation in a preclinical model of retinitis pigmentosa. Mol Ther : J Am Soc Gene Ther. 2016. https://doi.org/10.1038/mt.2016.107.
Long C., McAnally J. R., Shelton J. M., Mireault A. A., Bassel-Duby R., and Olson E. N., Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA, Science (New York, N.Y.), 2014, https://doi.org/10.1126/science.1254445.
Parikh BA, Beckman DL, Patel SJ, White JM, Yokoyama WM. Detailed phenotypic and molecular analyses of genetically modified mice generated by CRISPR-Cas9-mediated editing. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0116484.
Miao K, et al. Optimizing CRISPR/Cas9 technology for precise correction of the Fgfr3-G374R mutation in achondroplasia in mice. J Biol Chem. 2019. https://doi.org/10.1074/jbc.RA118.006496.
Gu B, Posfai E, Rossant J. Efficient generation of targeted large insertions by microinjection into two-cell-stage mouse embryos. Nat Biotechnol. 2018. https://doi.org/10.1038/nbt.4166.
Stamatiadis P. et al. Comparative analysis of mouse and human preimplantation development following POU5F1 CRISPR/Cas9 targeting reveals interspecies differences. Human Reprod(Oxford, England). 2021 https://doi.org/10.1093/humrep/deab027.
Wilde JJ, et al. Efficient embryonic homozygous gene conversion via RAD51-enhanced interhomolog repair. Cell. 2021. https://doi.org/10.1016/j.cell.2021.04.035.
Bischoff N, Wimberger S, Maresca M, Brakebusch C. Improving precise CRISPR genome editing by small molecules: is there a magic potion? Cells. 2020. https://doi.org/10.3390/cells9051318.
Ma H. et al., Ma et al. reply, Nature, 2018, https://doi.org/10.1038/s41586-018-0381-y.
Alanis-Lobato G, et al. Frequent loss of heterozygosity in CRISPR-Cas9-edited early human embryos. Proc Natl Acad Sci USA. 2021. https://doi.org/10.1073/pnas.2004832117.
Adikusuma F, et al. Large deletions induced by Cas9 cleavage. Nature. 2018. https://doi.org/10.1038/s41586-018-0380-z.
Papathanasiou S, et al. Whole chromosome loss and genomic instability in mouse embryos after CRISPR-Cas9 genome editing. Nat Commun. 2021. https://doi.org/10.1038/s41467-021-26097-y.
Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016. https://doi.org/10.1093/humupd/dmv040.
Cardona Barberan A., Boel A., Vanden Meerschaut F., Stoop D., and Heindryckx B., Fertilization failure after human ICSI and the clinical potential of PLCZ1, Reproduction (Cambridge, England), 2022, https://doi.org/10.1530/REP-21-0387.
Saunders C. M. et al., PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development, Development (Cambridge, England), 2002, https://doi.org/10.1242/dev.129.15.3533.
Yoon SY, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Investig. 2008. https://doi.org/10.1172/JCI36942.
Kashir J, Heindryckx B, Jones C, De Sutter P, Parrington J, Coward K. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010. https://doi.org/10.1093/humupd/dmq018.
Ferrer-Vaquer A, Barragan M, Freour T, Vernaeve V, Vassena R. PLCzeta sequence, protein levels, and distribution in human sperm do not correlate with semen characteristics and fertilization rates after ICSI. J Assist Reprod Genet. 2016. https://doi.org/10.1007/s10815-016-0718-0.
Heindryckx B., Van der Elst J., De Sutter P., and Dhont M., Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI, Human reproduction (Oxford, England), 2005, https://doi.org/10.1093/humrep/dei029.
Ferrer-Buitrago M, et al. Comparative study of preimplantation development following distinct assisted oocyte activation protocols in a PLC-zeta knockout mouse model. Mol Hum Reprod. 2020. https://doi.org/10.1093/molehr/gaaa060.
Bonte D, et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: a 17-year retrospective study. Fertil Steril. 2019. https://doi.org/10.1016/j.fertnstert.2019.04.006.
Hachem A. et al., PLCzeta is the physiological trigger of the Ca(2+) oscillations that induce embryogenesis in mammals but conception can occur in its absence, Development (Cambridge, England), 2017, https://doi.org/10.1242/dev.150227.
Fogarty NME, et al. Genome editing reveals a role for OCT4 in human embryogenesis. Nature. 2017. https://doi.org/10.1038/nature24033.
Peng C, et al. Accurate detection and evaluation of the gene-editing frequency in plants using droplet digital PCR. Front Plant Sci. 2020. https://doi.org/10.3389/fpls.2020.610790.
De Leeneer K, et al. Flexible, scalable, and efficient targeted resequencing on a benchtop sequencer for variant detection in clinical practice. Hum Mutat. 2015. https://doi.org/10.1002/humu.22739.
Almeida JL, et al. Interlaboratory study to validate a STR profiling method for intraspecies identification of mouse cell lines. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0218412.
Almeida JL, Hill CR, Cole KD. Mouse cell line authentication. Cytotechnology. 2014. https://doi.org/10.1007/s10616-013-9545-7.
Deleye L, et al. Shallow whole genome sequencing is well suited for the detection of chromosomal aberrations in human blastocysts. Fertil Steril. 2015. https://doi.org/10.1016/j.fertnstert.2015.07.1144.
Sante T, et al. ViVar: a comprehensive platform for the analysis and visualization of structural genomic variation. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0113800.
Bekaert B, Boel A, Cosemans G, De Witte L, Menten B, Heindryckx B. CRISPR/Cas gene editing in the human germline. Semin Cell Dev Biol. 2022. https://doi.org/10.1016/j.semcdb.2022.03.012.
Jayavaradhan R, et al. CRISPR-Cas9 fusion to dominant-negative 53BP1 enhances HDR and inhibits NHEJ specifically at Cas9 target sites. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-10735-7.
Nambiar TS, et al. Stimulation of CRISPR-mediated homology-directed repair by an engineered RAD18 variant. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-11105-z.
Paulsen BS, et al. Ectopic expression of RAD52 and dn53BP1 improves homology-directed repair during CRISPR-Cas9 genome editing. Nature biomedical engineering. 2017. https://doi.org/10.1038/s41551-017-0145-2.
Wienert B, et al. Timed inhibition of CDC7 increases CRISPR-Cas9 mediated templated repair. Nat Commun. 2020. https://doi.org/10.1038/s41467-020-15845-1.
Liang X, Potter J, Kumar S, Ravinder N, Chesnut JD. Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA. J Biotechnol. 2017. https://doi.org/10.1016/j.jbiotec.2016.11.011.
Paquet D, et al. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. 2016. https://doi.org/10.1038/nature17664.
Shin HY, et al. CRISPR/Cas9 targeting events cause complex deletions and insertions at 17 sites in the mouse genome. Nat Commun. 2017. https://doi.org/10.1038/ncomms15464.
Boutin J, et al. On-target adverse events of CRISPR-Cas9 nuclease: more chaotic than expected. The CRISPR journal. 2022. https://doi.org/10.1089/crispr.2021.0120.
Singh P, Schimenti JC, Bolcun-Filas E. A mouse geneticist’s practical guide to CRISPR applications. Genetics. 2015. https://doi.org/10.1534/genetics.114.169771.
Bae S., Park J., and Kim J. S., Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases, Bioinformatics (Oxford, England), 2014, https://doi.org/10.1093/bioinformatics/btu048.
Borgstrom E, Paterlini M, Mold JE, Frisen J, Lundeberg J. Comparison of whole genome amplification techniques for human single cell exome sequencing. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0171566.
Volozonoka L, Miskova A, Gailite L. Whole genome amplification in preimplantation genetic testing in the era of massively parallel sequencing. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23094819.
Cardona Barberan A., Boel A., Vanden Meerschaut F., Stoop D., and Heindryckx B., Sperm factors and egg activation: fertilization failure after human ICSI and the clinical potential of PLCZ1, Reproduction (Cambridge, England), 2022, https://doi.org/10.1530/REP-21-0387.
Xin A, et al. Disruption in ACTL7A causes acrosomal ultrastructural defects in human and mouse sperm as a novel male factor inducing early embryonic arrest. Sci Adv. 2020. https://doi.org/10.1126/sciadv.aaz4796.
Acknowledgements
The figures were created with BioRender.com. Graphs were created with GraphPad Prism.
Funding
We want to acknowledge the financial support by FWO-Vlaanderen (Flemish fund for scientific research) for B.B. (11C2821N), A.B. (1298722N), G.C. (11L8822N), B.M. (G077422N) and B.H. (G077422N).
Author information
Authors and Affiliations
Contributions
B.B., A.B., B.M. and B.H. conceived and designed the project. B.B. performed most of the experiments, data analysis and wrote the manuscript. J.P. previously generated the mouse model and provided sperm samples. B.B., A.B., B.M. and P.C. designed and/or performed the genetic analyses. A.R. performed the CRISPR/Cas9 mouse germline injections. G.C. and S.D. helped with the experimental set-up. B.B., A.B., S.M.C.S.L, D.S., B.M., P.C. and B.H. interpreted the data. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
All procedures involving live-animal handling and euthanasia of the animals were approved by the Ghent University Hospital Ethical Committee for Laboratory Animals (ECD 19-107 and ECD 19-107aanp) and were conform to all relevant regulatory standards.
Consent
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bekaert, B., Boel, A., Rybouchkin, A. et al. Various repair events following CRISPR/Cas9-based mutational correction of an infertility-related mutation in mouse embryos. J Assist Reprod Genet (2024). https://doi.org/10.1007/s10815-024-03095-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10815-024-03095-9