Abstract
Background
The impact of vitrification on oocyte developmental competence as a function of donor age remains an important issue in assisted reproductive technologies (ARTs).
Methods
Equine germinal vesicle (GV) or metaphase II (M(II) oocytes were vitrified using the Cryotop® method. Spindle organization and chromosome alignment were evaluated from confocal imaging data sets of in vivo (IVO) or in vitro (IVM) matured oocytes subjected to vitrification or not. Intracytoplasmic sperm injection (ICSI) from the same groups was used to assess developmental potential.
Results
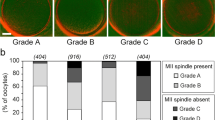
An increase in chromosome misalignment was observed in spindles from older mares when compared to those of younger mares (P < 0.05). When MII oocytes subjected to vitrification were examined following warming, there was no difference in the percentage of oocytes displaying chromosome misalignment. Next, GV oocytes, collected from the ovaries of younger and older mares, were compared between fresh IVM and IVM following vitrification and warming. For nonvitrified samples, an age difference was again noted for spindle organization and chromosome alignment, with a higher (P < 0.05) percentage of normal bipolar meiotic spindles with aligned chromosomes observed in nonvitrified oocytes from young versus older mares. Vitrification led to a reduction of spindle length (P < 0.05) for oocytes from old mares, whether vitrified at GV or MII stages, whereas this effect was not observed in oocytes from young mares except those vitrified at GV and subjected to IVM. Oocyte developmental potential after vitrification was evaluated after ICSI of vitrified and warmed MII or GV oocytes from young mares. From 25 MII oocytes, 18 oocytes were injected with sperm, and six blastocysts were produced, which, upon transfer to mares’ uteri, resulted in four pregnancies. Immature (GV) oocytes collected from live mares were also vitrified, warmed, and matured in vitro before ICSI. In this group, nonvitrified, control, and vitrified oocytes did not differ (P > 0.05) with respect to the incidence of maturation to MII, cleavage after ICSI, or blastocyst development.
Conclusion
These findings demonstrate an effect of maternal age in an equine model at the level of meiotic spindle integrity and chromosome positioning that is influenced by both the meiotic stage at which oocytes are vitrified and whether meiotic maturation occurred in vivo or in vitro.





Similar content being viewed by others
References
Altermatt JL, Suh TK, Stokes JE, Carnevale EM. Effects of age and eFSH on collection and viability of equine oocytes assessed by morphology and developmental competency after ICSI. Reprod Fertil Dev. 2009;21(4):615–23.
Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76(6):9–957.
Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11(10):2217–22.
Bogliolo L, Murrone O, Piccinini M, Ariu F, Ledda S, Tilocca S, Albertini DF. Evaluation of the impact of vitrification on the actin cytoskeleton of in vitro matured ovine oocytes by means of Raman microspectroscopy. J Assist Reprod Genet. 2015;32(2):185–9.
Boiso I, Marti M, Santalo J, Ponsa M, Barri PN, Veiga A. A confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod. 2002;17:1885–91.
Bromfield JJ, Coticchio G, Hutt K, Sciajno R, Borini A, Albertini DF. (2009). Meiotic spindle dynamics in human oocytes following slow-cooling cryopreservation. Hum Reprod. 2009;24(9):2114–23.
Canesin HS, Brom-de-Luna JG, Choi YH, , Ortiz I, Diaw M, & Hinrichs K. (2017). Blastocyst development after intracytoplasmic sperm injection of equine oocytes vitrified at the germinal vesicle stage. Cryobiology 75 (2017) 52-59.
Canesin HS, Brom-de-Luna JG, Choi Y-H, Hinrichs K. Toxicity of a vitrification protocol for equine immature oocytes. J Equine Vet Sci. 2016;47:79–80.
Carboni S, Rosati I, Maclellan LJ, Ariu F, Bogliolo L, Zedda MT, Pau S, Carnevale EM, Ledda S. Vitrification of GV and IVM horse oocytes with two different equilibration methods. In: Proceedings of 10th Congress of Italian Society of Animal Reproduction (SIRA 2012); 2012. p. 12–3.
Carnevale EM. The mare model for follicular maturation and reproductive aging in the woman. Theriogenology. 2008;69:23–30.
Carnevale EM. The mare as an animal model for reproductive aging in the woman. In: Animal Models and Human Reproduction (H Schatten and GM Constantinescu). Hoboken: Wiley-Blackwell; 2017. p. 235–45.
Carnevale EM, Ginther OJ. Defective oocytes as a cause of subfertility in old mares. Biol Reprod. 1995;1(Equine Reproduction VI):209–14.
Carnevale EM, Uson M, Bozzola JJ, King SS, Schmitt SJ, Gates HD. Comparison of oocytes from young and old mares with light and electron microscopy. Theriogenology. 1999;51:299.
Carnevale EM, Maclellan LJ, Coutinho da Silva MA, Scott TJ, Squires EL. Comparison of culture and insemination techniques for equine oocyte transfer. Theriogenology. 2000;54(6):981–7.
Carnevale EM, Maclellan LJ, Countinho da Silva MA, Squires EL. Pregnancies after collection and transfer of oocytes from ovaries of five euthanized mares. J Am Vet Med Assoc. 2003;222(1):60–2.
Carnevale EM. Advances in collection, transport and maturation of equine oocytes for assisted reproductive techniques. Vet Clin North Am Equine Pract. 2016;32(3):379–99.
Cheng J, Jia B, Wu T, Zhou G, Hou Y, Fu X, Zhu S. Effects of vitrification for germinal vesicle and metaphase II oocytes on subsequent centromere cohesion and chromosome aneuploidy in mice. Theriogenology. 2014;82(3):495–500.
Chen SU, Lien YL, Chen HF, Chao KH, Ho HN, Yang YS. Open pulled straws for vitrification of mature mouse oocytes preserve patterns of meiotic spindles and chromosomes better than conventional straws. Hum Reprod. 2000;15:2598–603.
Chen CK, Wang CW, Tsai WJ, Hsieh LL, Wang HS, Soong YK. Evaluation of meiotic spindles in thawed oocytes after vitrification using polarized light microscopy. Fertil Steril. 2004;82(3):666–72.
Clerico G, Taminelli G, Veronesi JC, Polola J, Pagura N, Pinto C, Sansinena M. Mitochondrial function, blastocyst development and live foals born after ICSI of immature vitrified/warmed equine oocytes matured with or without melatonin. Theriogenology. 2021;160:40–9.
Cobo A, Bellver J, Domingo J, Pérez S, Crespo J, Pellicer A, Remohí J. New options in assisted reproduction technology: the Cryotop method of oocyte vitrification. Reprod Biomed Online. 2008;17(1):68–72.
Coticchio G, Bromfield JJ, Sciajno R, Gambardella A, Scaravelli G, Borini A, Albertini DF. Vitrification may increase the rate of chromosome misalignment in the metaphase II spindle of human mature oocytes. Reprod Biomed Online. 2009;19(Suppl 3):29–34.
de Leon PM, Campos VF, Corcini CD, Santos EC, Rambo G, Lucia T Jr, Deschamps JC, Collares T. (2012). Cryopreservation of immature equine oocytes, comparing a solid surface vitrification process with open pulled straws and the use of a synthetic ice blocker. Theriogenology. 2012;77(1):21–7.
Ducheyne KD, Rizzo M, Beitsma M, Deelan C, Daels PF, Stout TAE, de Ruijter-Viallani M. Vitrifying equine oocytes at the germinal vesicle stage disturbs spindle morphology and chromosome alignment. J Equine Vet. 2018;66:178.
Ducheyne KD, Rizzo M, Daels PF, Stout TAE, de Ruijter-Villani M. Vitrifying immature equine oocytes impairs their ability to correctly align the chromosomes on the MII spindle. Reprod Fertil Dev. 2019;31(8):1330–8.
Foss R, Ortis H, Hinrichs K. Effect of potential oocyte transport protocols on blastocyst rates after intracytoplasmic sperm injection in the horse. Equine Vet J. 2013;45(Suppl. 45):39–43.
Ginther OJ. The mare: a 1000-pound guinea pig for study of the ovulatory follicular wave in women. Theriogenology. 2012;77:18–828.
Ginther OJ. Reproductive biology of the mare: basic and applied aspects. 2nd ed. Cross Plains: Equiservices; 1992.
Hinrichs K, Schmidt AL, Friedman PP, Selgrath JP, Martin MG. In vitro maturation of horse oocytes: characterization of chromatin configuration using fluorescence microscopy. Biol Reprod. 1993;48(2):363–70.
Hinrichs K, Schmidt AL. Meiotic competence in horse oocytes: interactions among chromatin configuration, follicle size, cumulus morphology, and season. Biol Reprod. 2000;62:1402–8.
Hinrichs K, Choi Y-H, Norris JD, Love LB, Bedford-Guaus SJ, Hartman DL, Velez IC. Evaluation of foal production following intracytoplasmic sperm injection and blastocyst culture of oocytes from ovaries collected immediately before euthanasia or after death of mares under field conditions. J Amer Vet Med Assoc. 2012;241(8):1070–4.
Hochi S, Kozawa M, Fujimoto T, Hondo E, Yamada J, Oguri N. In vitro maturation and transmission electron microscopic observation of horse oocytes after vitrification. Cryobiology. 1996;33(3):300–10.
Hurtt AE, Landim-Alvarenga F, Seidel GE Jr, Squires EL. Vitrification of immature and mature equine and bovine oocytes in an ethylene glycol, ficoll and sucrose solution using open-pulled straws. Theriogenology. 2000;54:119–28.
Kuwayama M, Vajta G, Leda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005a;11(5):608–14.
Kuwayama M, Vatja G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005b;11(3):300–8.
Maclellan LJ, Carnevale EM, Coutinho da Silva MA, Scoggin CF, Bruemmer JE, Squires EL. Pregnancies from vitrified equine oocytes collected from super-stimulated and non-stimulated mares. Theriogenology. 2002;58(5):911–9.
Maclellan LJ, Stokes JE, Preis KA, McCue PM, Carnevale EM. Vitrification, warming, ICSI and transfer of equine oocytes matured in vivo. Anim Reprod Sci. 2010;121:S260–1.
Madill S. Reproductive considerations: mare and stallion. Veterinary Clinics Equine. 2002;18:591–619.
Messinger SM, Albertini DF. Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100(Pt 2):289–98.
Ortiz-Escribano N, Bogado Pascottini O, Woelders H, Vandenberghe L, De Schauwer C, Govaere J, Van den Abbeel E, Vullers T, Ververs C, Roels K, Van De Velde M, Van Soom A, Smits K. An improved vitrification protocol for equine immature oocytes, resulting in a first live foal. Equine Vet J. 2018;50(3):391–7.
Park SE, Son WY, Lee SH, Lee KA, Ko JJ, Cha KY. Chromosome and spindle configurations of human oocytes matured in vitro after cryopreservation at the germinal vesicle stage. Fertil Steril. 1997;68:920–6.
Parks JE, Ruffing NA. Factors affecting low temperature survival of mammalian oocytes. Theriogenology. 1992;37:59–73.
Rader K, Choi YH, Hinrichs K. Intracytoplasmic sperm injection, embryo culture, and transfer of in vitro–produced blastocysts. Vet Clin Equine. 2016;32:401–13.
Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review of meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–55.
Rizzo M, Kops GJPL, Deelan C, Beitsma M, Cristarella S, Stout TAE, de Ruijter-Viallani M. Compromised spindle assembly checkpoint function in oocytes from aged mares impairs correct chromosome alignment. J Equine Vet. 2018;66:177.
Rojas C, Palomo MJ, Albarracín JL, Mogas T. Vitrification of immature and in vitro matured pig oocytes: study of distribution of chromosomes, microtubules, and actin microfilaments. Cryobiology. 2004;49(3):211–20.
Rosati I, Maclellan LJ, Ariu F, Bogliolo L, Zedda MT, Pau S, Carnevale EM, Ledda S. Vitrification of GV and IVM horse oocytes with two different equilibration methods. Reprod Domest Anim. 2012;47(Suppl. 4):508.
Ruggeri E, DeLuca KF, Galli C, Lazzari G, DeLuca JG, Stokes JE, Carnevale EM. Use of confocal microscopy to evaluate equine zygote development after sperm injection of oocytes matured in vivo or in vitro. Microsc Microanal. 2017; https://doi.org/10.1017/S1431927617012740. published online: 06 Dec 2017 ahead of print
Saunders KM, Parks JE. Effects of cryopreservation procedures on the cytology and fertilization rate of in vitro-matured bovine oocytes. Biol Reprod. 1999;61:178–87.
Schatten H, Sun QY. Centrosome dynamics during mammalian oocyte maturation with a focus on meiotic spindle formation. Mol Reprod Dev. 2011;78:757–68.
Scoggin CF. Not just a number: effect of age on fertility, pregnancy and offspring vigour in thoroughbred brood-mares. Reprod Fertil Dev. 2015;27:872–9.
Tharasanit T, Colleoni S, Galli C, Colenbrander B, Stout TA. Protective effects of the cumulus-corona radiata complex during vitrification of horse oocytes. Reproduction. 2009;137(3):391–401.
Tharasanit T, Colenbrander B, Stout TAE. Effect of maturation stage at cryopreservation on post-thaw cytoskeleton quality and fertilizability of equine oocytes. Mol Reprod Dev. 2006a;73:627–37.
Tharasanit T, Colleoni S, Lazzari G, Colenbrander B, Galli C, Stout TA. (2006b) Effect of cumulus morphology and maturation stage on the cryopreservability of equine oocytes. Reproduction. 2006;132(5):759–69.
Tremoleda JL, Van Haeften T, Stout TA, Colenbrander B, Bevers MM. Cytoskeleton and chromatin reorganization in horse oocytes following intracytoplasmic sperm injection: patterns associated with normal and defective fertilization. Biol Reprod. 2003;69(1):186–94.
Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65(1):236–44.
Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, Callesen H. Open Pulled Straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev. 1998;51(1):53–8.
Acknowledgements
This study was supported by the Cecil and Irene Hylton Family Foundation. Dr. Raul Gonzalez-Castro assisted with statistical analyses. Dr. Elena Ruggeri provided measurements for experiment 1A, and Drs. Jennifer Hatzel and Fabio Amoroso aspirated immature oocytes in experiment 2B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maclellan, L.J., Albertini, D.F., Stokes, J.E. et al. Use of confocal microscopy and intracytoplasmic sperm injection (ICSI) to assess viability of equine oocytes from young and old mares after vitrification. J Assist Reprod Genet 40, 2565–2576 (2023). https://doi.org/10.1007/s10815-023-02935-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02935-4