Abstract
Purpose
To analyze factors affecting segregation and ploidy results from Robertsonian carriers, and determine chromosomes involved impact chromosome stability during meiosis and mitosis.
Methods
This retrospective study include 928 oocyte retrieval cycles from 763 couples with Robertsonian translocations undergoing preimplantation genetic testing for structural rearrangements (PGT-SR) using next-generation sequencing (NGS) between December 2012 and June 2020.The segregation patterns of the trivalent of 3423 blastocysts were analyzed according to the carrier’s sex and age. A total of 1492 couples who received preimplantation genetic testing for aneuploidy (PGT-A) were included as the control group and matched according to maternal age and testing time stage.
Results
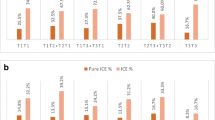
A total of 1728 (50.5%) normal/balanced embryos were identified from 3423 embryos diagnosed. The rate of alternate segregation in male Robertsonian translocation carriers was significantly higher than that in female carriers (82.3% vs. 60.0%, P < 0.001). However, the segregation ratio exhibited no difference between young and older carriers. Further, increasing maternal age decreased the proportion of transferable embryo cycle in both female and male carriers. And the ratio of chromosome mosaic from the Robertsonian translocation carrier group was significantly higher than that in the PGT-A control group (1.2% vs. 0.5%, P < 0.01).
Conclusions
The meiotic segregation modes were affected by the carrier sex and were independent of the carrier’s age. Advanced maternal age decreased the probability of obtaining a normal/balanced embryo. In additional, the Robertsonian translocation chromosome could increase the possibility of chromosome mosaicism during mitosis in blastocysts.


Similar content being viewed by others
References
Fryns JP, Van Buggenhout G. Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol. 1998;81:171–6.
Gardner RJM, Sutherland GR, Shaffer LG. Chromosome abnormalities and genetic counseling. 4th ed. Oxford; New York: Oxford University Press; 2012.
Stern C, Pertile M, Norris H, Hale L, Baker HW. Chromosome translocations in couples with in-vitro fertilization implantation failure. Hum Reprod. 1999;14:2097–101.
Therman E, Susman B, Denniston C. The nonrandom participation of human acrocentric chromosomes in Robertsonian translocations. Ann Hum Genet. 1989;53:49–65.
Yoshida A, Miura K, Shirai M. Cytogenetic survey of 1,007 infertile males. Urol Int. 1997;58:166–76.
Escudero T, Abdelhadi I, Sandalinas M, Munne S. Predictive value of sperm fluorescence in situ hybridization analysis on the outcome of preimplantation genetic diagnosis for translocations. Fertil Steril. 2003;79(Suppl 3):1528–34.
Guttenbach M, Engel W, Schmid M. Analysis of structural and numerical chromosome abnormalities in sperm of normal men and carriers of constitutional chromosome aberrations. A review. Hum Genet. 1997;100:1–21.
Mau-Holzmann UA. Somatic chromosomal abnormalities in infertile men and women. Cytogenet Genome Res. 2005;111:317–36.
Pylyp LY, Zukin VD, Bilko NM. Chromosomal segregation in sperm of Robertsonian translocation carriers. J Assist Reprod Genet. 2013;30:1141–5.
Fischer J, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2010;94:283–9.
Otani T, Roche M, Mizuike M, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod Biomed Online. 2006;13:869–74.
Jin H, Ping L, Jie Q, Ying L, Yongjian C. Translocation chromosome karyotypes of the Robertsonian translocation carriers’ embryos. Fertil Steril. 2010;93:1061–5.
Ko DS, Cho JW, Lee HS, Kim JY, Kang IS, Yang KM, et al. Preimplantation genetic diagnosis outcomes and meiotic segregation analysis of Robertsonian translocation carriers. Fertil Steril. 2013;99:1369–76.
Zhang L, Jiang W, Zhu Y, Chen H, Yan J, Chen ZJ. Effects of a carrier's sex and age on the segregation patterns of the trivalent of Robertsonian translocations. J Assist Reprod Genet. 2019;36:1963–9.
Miller DE. The interchromosomal effect: different meanings for different organisms. Genetics. 2020;216:621–31.
Gianaroli L, Magli MC, Ferraretti AP, Munne S, Balicchia B, Escudero T, et al. Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod. 2002;17:3201–7.
Mateu-Brull E, Rodrigo L, Peinado V, Mercader A, Campos-Galindo I, Bronet F, et al. Interchromosomal effect in carriers of translocations and inversions assessed by preimplantation genetic testing for structural rearrangements (PGT-SR). J Assist Reprod Genet. 2019;36:2547–55.
Munne S, Escudero T, Fischer J, Chen S, Hill J, Stelling JR, et al. Negligible interchromosomal effect in embryos of Robertsonian translocation carriers. Reprod Biomed Online. 2005;10:363–9.
Xie Y, Xu Y, Wang J, Miao B, Zeng Y, Ding C, et al. Preliminary analysis of numerical chromosome abnormalities in reciprocal and Robertsonian translocation preimplantation genetic diagnosis cases with 24-chromosomal analysis with an aCGH/SNP microarray. J Assist Reprod Genet. 2018;35:177–86.
Alfarawati S, Fragouli E, Colls P, Wells D. Embryos of Robertsonian translocation carriers exhibit a mitotic interchromosomal effect that enhances genetic instability during early development. PLoS Genet. 2012;8:e1003025.
Anton E, Vidal F, Blanco J. Interchromosomal effect analyses by sperm FISH: incidence and distribution among reorganization carriers. Syst Biol Reprod Med. 2011;57:268–78.
McGowan-Jordan J, Hastings R, Moore S. Re: International system for human cytogenetic or cytogenomic nomenclature (ISCN): some thoughts, by T. Liehr. Cytogenet Genome Res. 2021;161:225–6.
Zhou S, Cheng D, Ouyang Q, Xie P, Lu C, Gong F, et al. Prevalence and authenticity of de-novo segmental aneuploidy (>16 Mb) in human blastocysts as detected by next-generation sequencing. Reprod Biomed Online. 2018;37:511–20.
Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981;58:282–5.
Xie P, Hu L, Peng Y, Tan YQ, Luo K, Gong F, et al. Risk factors affecting alternate segregation in blastocysts from preimplantation genetic testing cycles of autosomal reciprocal translocations. Front Genet. 2022;13:880208.
Lane S, Kauppi L. Meiotic spindle assembly checkpoint and aneuploidy in males versus females. Cell Mol Life Sci. 2019;76:1135–50.
Eaker S, Pyle A, Cobb J, Handel MA. Evidence for meiotic spindle checkpoint from analysis of spermatocytes from Robertsonian-chromosome heterozygous mice. J Cell Sci. 2001;114:2953–65.
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656–63.e1.
Akera T, Trimm E, Lampson MA. Molecular Strategies of Meiotic Cheating by Selfish Centromeres. Cell. 2019;178:1132–44.e10.
Cavazza T, Takeda Y, Politi AZ, Aushev M, Aldag P, Baker C, et al. Parental genome unification is highly error-prone in mammalian embryos. Cell. 2021;184:2860–77. e22.
Klaasen SJ, Truong MA, van Jaarsveld RH, Koprivec I, Stimac V, de Vries SG, et al. Nuclear chromosome locations dictate segregation error frequencies. Nature. 2022;607:604–9.
Acknowledgements
We are grateful to the staff of the PGT and IVF group in CITIC XIANGYA for their technical assistance.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant 81873478, to L.H.) and Natural Science Foundation of Hunan Province (grant 2022JJ30414 and 2019 JJ 50397).
Author information
Authors and Affiliations
Contributions
GL, GL, and PX designed the experiments and drafted the manuscript; GF, KL, YQT, LH, and YT contributed to the clinical samples; PX, TD, and ZZ, performed the experiments and analyzed the data; PX, TD, and ZZ, reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dang, T., Xie, P., Zhang, Z. et al. The effect of carrier characteristics and female age on preimplantation genetic testing results of blastocysts from Robertsonian translocation carriers. J Assist Reprod Genet 40, 1995–2002 (2023). https://doi.org/10.1007/s10815-023-02853-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02853-5