Abstract
The rapid outbreak of the coronavirus disease 2019 (COVID-19) pandemic has brought challenges to different medical fields, especially reproductive health. To date, most studies on the effects of COVID-19 on male reproduction have some limitations. In addition, there is little research on the mechanisms underlying by which severe acute respiratory syndrome coronavirus 2 infection affects semen quality. Here, we revealed the possible impact of COVID-19 on sperm parameters and the potential mechanisms. At present, it is still controversial whether COVID-19-induced fever adversely affects sperm parameters. Severe acute respiratory syndrome coronavirus 2 can induce up-regulation of pro-inflammatory cytokine, which leads to the destruction of blood-testis barrier and impairment of spermatogenesis. Moreover, severe viral infection of the respiratory system could induce systemic oxidative stress. Sperm are highly vulnerable to it due to their limited levels of antioxidant defense, unsophisticated DNA damage detection and repair mechanisms. Our review prompt medical staff and patients to consciously check the reproductive function of COVID-19 male patients. Moreover, opening our prospective beyond the direct infection could be the key to better understand the COVID-19 short and long-term effects and provide a new idea for future treatment of patients with reproductive function injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In December 2019, China has reported a group of pneumonia cases of unknown etiology to the World Health Organization. A novel β-coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected in samples taken from the patient's lower respiratory tract. According to the statistics of the World Health Organization, as of March 21, 2023, coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has led to 761 million confirmed cases and 6.88 million deaths, which has significantly stressed public health systems worldwide [1]. Like SARS-CoV-1, SARS-CoV-2 infects host cells with transmembrane serine protease 2 and receptor angiotensin-converting enzyme 2 (ACE2) [2]. Notably, ACE2 is expressed in many tissues including the cardiovascular system, gastrointestinal tract and liver in addition to the lungs. Correspondingly, damage to these organs has been observed in patients with COVID-19 [3,4,5].
The human male reproductive system is vulnerable to virus infection because the blood-testis barrier cannot completely prevent the virus. Up to now, a variety of viruses that can induce orchitis and lead to male infertility have been detected in human semen (e.g., mumps virus, Zika virus, human immunodeficiency virus etc.), but the picture is less complete for COVID-19 [6]. Seminiferous duct cells, renal tubular cells, Leydig and Sertoli cells show a high level of ACE2 expression, which suggests that the testis could be a potential target for direct damage by SARS-CoV-2 [7]. In addition, the testicular expression of ACE2 is age related. Specifically, ACE2 is highly expressed in patients aged 20-30 years, while it is extremely low in patients aged 60 years [8]. Thus, male reproductive health during the COVID-19 pandemic has aroused widespread concern. However, there have been some limitations such as small sample size and inadequate study design in some studies on the effects of COVID-19 on male reproduction [9]. In addition, most studies conducted in this area did not present pre-infection data as a reference point and, instead, rely on cross-sectional comparisons with uninfected controls. Moreover, there have been few studies on the mechanisms underlying the observed impacts of SARS-CoV-2 infection on semen quality. Here, based on the available evidence, we included controlled before-and-after trials to discuss the changes in sperm parameters and further explored the mechanism of the effects of COVID-19 on male reproduction in terms of fever, inflammation and oxidative stress (OS). This helps clinicians assess male fertility in COVID-19 positive or recovered patients and provides guidance for the andrology treatment.
Changes of semen parameters in COVID-19 cases
In our previous systematic review, we reviewed the effect of COVID-19 on male fertility [10]. However, majority of included studies were difficult to use the self-controlled study design to eliminate confounding factors and selection bias since most subjects in these studies underwent semen analysis only once. To obtain more accurate data, all the studies we included in this review were controlled before-and-after trials.
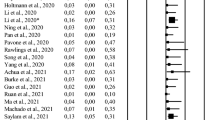
[Table 1 summarizes the changes in semen parameters of subjects before and after SARS-CoV-2 infection [11,12,13,14,15]. Most patients diagnosed with COVID-19 showed decrease in total motility of sperm. At the same time, the sperm progressive motility, concentration and volume also showed significant reduce in some semen samples. Furthermore, it appears to correlate to the disease severity. Specifically, all sperm parameters were significantly reduced in the moderate symptom group, while only the progressive and total motility were decreased in the mild symptom group [14].
Possible mechanisms of COVID-19 affecting male reproduction
We have summarized that SARS-CoV-2 was not found in semen samples of COVID-19 patients in most studies [16]. Even several studies described the presence of SARS-CoV-2, it may be because the samples are not collected under strictly sterile conditions, and thus be contaminated with aerosols or other body fluids from the patients [17]. Therefore, the semen injury described above is probably caused by the coexistence of several biological mechanisms that synergistically interfere with the reproductive system instead of direct infection.
Fever
Fever has been widely reported as one of the most common clinical manifestations of COVID-19 patients. For example, a study of 4203 patients mostly from China identified that 80.5% were diagnosed with fever [18]. Moreover, the estimated duration of fever in patients with SARS-CoV-2 is longer than those with MERS and other virus diseases [19,20,21]. Thus, COVID-19-induced fever may have a greater impact.
The process of spermatogenesis is temperature-dependent and occurs optimally when the temperature is slightly lower than that of the body. Although the testis contains heat shock proteins that resist high temperatures and chemical radiation, changes in its temperature occur when the body is in a state of high heat for a long time [22]. Jannes et al. observed that both relative and absolute testis weight decreased following scrotal heating [23]. Moreover, upon prolongation, the rate of cell cycle disruption is higher than the production of heat shock proteins, hence inducing spermatocyte apoptosis [24]. Several reports have found that an acute fever of 39-40 °C for 1-4 days could cause changes in the sperm parameters such as vitality, total sperm count, and DNA fragmentation [25, 26]. Thus, it is easy to understand that males showed a period of infertility for 10-32 days after testicular heating [27]. Temiz et al. demonstrated a significant decline in the percentage of normal morphology of the semen in COVID-19 patients and speculated that this was caused by fever [28]. Meanwhile, the fever-positive group showed significantly lower complete sperm motility and concentration than the fever-negative group [29]. However, Erbay et al. found that sperm motility and vitality decreased in both COVID-19 patients with and without fever. Moreover, it was observed that fever did not contribute to this condition [13]. Data derived from two other studies also supported this thought [12, 30]. There are several points to consider when interpreting these divergent results. First, the sample size was limited and most of the patients had only one semen sample collection performed after infection. In addition, there may have been differences in the degree of fever in the populations recruited for these studies. Thus, whether the effect of COVID-19 on sperm parameters is caused by fever remains controversial, and more detailed grouping studies of larger populations are necessary in the future.
Inflammation
The blood-testis barrier provides an isolated immune-privileged microenvironment for sperm, which can actively exclude immune cells and other factors from entering the seminiferous tubules and being exposed to developing germ cells [31]. However, despite the privileged immune status, testis cannot be protected from the general immune response. Histopathological changes including inflammatory damages of seminiferous tubules with interstitial edema, congestion, inflammatory cell infiltration and erythrocyte exudation were observed in COVID-19 patients in two separate reports, suggesting that COVID-19 might damage the male reproductive system by inflammatory [32, 33]. As a substantial systemic inflammatory syndrome, hemophagocytic lymphohistiocytosis is a recognized mechanism that appears to contribute to tissue damage and multiple organ dysfunction in COVID-19 patients. This immunologically induced multi-organ damage includes testicular damage, which may have a negative effect on spermatogenesis, thus adversely affecting the quality of semen and male fertility [34]. Moreover, infiltration of leukocytes, CD68+ macrophages and CD3+ T lymphocytes into the testicular interstitial tissue can produce interferons that may decrease the production of testosterone, which is also associated with abnormal spermatogenesis [35,36,37]. Notably, the plasma levels of cytokines (IL-2, IL-6, IL-7, IL-10, TNF-α and MCP-1) are higher in patients with severe SARS-CoV-2 infection [3, 38]. Available evidence suggests that the imbalance between these pro- and anti-inflammatory molecules, such as IL-6 and TNF-α, in testis cells can result in orchitis [39]. However, orchitis is not a common symptom in male patients with COVID-19 (8.82%, 50/567) (Table 2) [15, 29, 40,41,42,43,44,45,46,47,48,49,50]. This may be due to the immunosuppressive properties of Sertoli cells, which plays a key role in suppressing inflammation and limiting virus-associated testicular damage to a certain extent [51]. Notably, 74 (42.05%) showed an abnormal value for at least one sperm parameter among 176 male COVID-19 patients, which was much higher than the incidence of orchitis (Table 2). Thus, it is reasonable to speculate that orchitis is not the entire cause of abnormal sperm parameters.
Oxidative stress
Most mechanisms of tissue damage caused by SARS-CoV-2 infection are directly associated with OS, which occurs when the balance between oxidants and antioxidants is disrupted towards an overabundance of reactive oxygen species (ROS) [52, 53]. Indeed, ROS production was significantly elevated in COVID-19 patients with a median of nine times higher than in healthy control group and was particularly high in severe cases [54]. At normal physiological levels, ROS mediate important physiological mechanisms such as sperm maturation, capacitation, acrosome reaction, and fertilization by regulating intracellular signaling cascades [55]. However, excessive ROS have adverse effects on the proteins and lipids of the sperm plasma membrane as well as induce the breakdown of sperm DNA [56]. This probably because the cell membranes of sperm are rich in polyunsaturated fatty acids that are extremely vulnerable to ROS-induced lipid peroxidation [57]. Any damage to the cell membrane consequently disturbs its fluidity, leading to impaired sperm motility and dysregulated events of membrane fusion during the acrosome reaction and fertilization [58]. OS status in the COVID-19 patients was remarkably changed as evidenced by increased levels of lipid peroxidation [59]. It promotes destruction of membrane fluidity and rapid loss of sperm adenosine triphosphate, leading to midpiece defects, reduced sperm motility and viability [60]. In addition, ROS-induced DNA damage was found in both the nuclear and mitochondrial genomes of sperm [61]. Human telomeres contain many repeats of the guanine rich hexamer, which are preferential targets of free-radical attack owing to the fact that guanine is more susceptible to OS than other nucleotides. Oxidative damage to telomeres usually results in the generation of highly mutagenic 8-hydroxy-2-deoxyguanosine adducts [62]. When paired with adenine, it forms a stable Hoogsteen mispair containing two hydrogen bonds by adopting the syn conformation around the N-glycosylic bond. This results in G: C to T: A inversion during DNA replication and leads to single-strand and double-strand breaks [63,64,65]. Moreover, mitochondrial DNA is susceptible to oxidative damage caused by OS due to the lack of protective histones, DNA-binding proteins and effective repair systems [66]. In the meanwhile, dysfunctional mitochondria produce less adenosine triphosphate levels which can inhibit the progression of stem cell precursors during the cell cycle, leading to hypospermatogenesis or maturation arrest [67]. These OS-induced damages, especially sperm DNA fragmentation may adversely affect reproductive outcomes because the inherent integrity of DNA determines the success rate of fertilization and embryonic development [68]. Generally speaking, DNA fragmentation index <30% is considered a necessary prerequisite for optimal fertility. Falahieh et.al detected that it was 33.10% in samples from COVID-19 patients on day 14 after diagnosis. Moreover, sperm total motility and progressive motility were below the normal range. Fortunately, the ROS concentrations decreased at 120 days after the COVID-19 diagnosis compared with the acute phase, accompanied by improvements in DNA fragmentation index and sperm motility [69]. Thus, the increases in semen ROS levels play a key role in the adverse effects of SARS-CoV-2 infection on sperm parameters, but the damage will gradually decrease over time.
Conclusions
Given the limitations of previous studies, we included controlled before-and-after studies to get more accurate data on the effects of SARS-CoV-2 infection on male reproduction. The results showed that patients diagnosed with COVID-19 had a significant decrease in sperm parameters (sperm concentration, volume, progressive motility and total motility). Moreover, the magnitude of the decline is related to the severity of the disease. Given that SARS-CoV-2 viral RNA was not found in semen samples from most COVID-19 patients, these changes may be caused by several biological mechanisms that synergistically interfere with the reproductive system rather than direct viral infection. COVID-19-induced fever was once thought to be one of the important causes, but the extent to which fever plays a role in this remains to be further investigated. Systemic or local inflammation in COVID-19 patients can cause hemophagocytic lymphohistiocytosis and upregulation of cytokines, thus leading to testicular damage and abnormal spermatogenesis. Notably, the prevalence of abnormal sperm parameters was higher than the incidence of orchitis in COVID-19 patients. Thus, the orchitis is not the only cause of abnormal sperm parameters. Another concern about how COVID-19 affects male fertility is OS. Recent study has indicated that excessive ROS can result in decreased sperm motility and sperm maturation arrest by lipid peroxidation of sperm membrane as well as sperm DNA damage. Therefore, infected males should be examined for fertility as soon as possible after recovery, particularly if they are married and wish to have children. Moreover, further studies are needed to assess the exact mechanisms by which COVID-19 affects the male reproductive system and fertility and to estimate the reversibility of its long-term effects.
References
"WHO Coronavirus (COVID-19) Dashboard." https://covid19.who.int/. (Accessed 24 Mar 2023).
Tian Y, Zhou LQ. Evaluating the impact of COVID-19 on male reproduction. Reproduction. 2021;161(2):R37–44. https://doi.org/10.1530/REP-20-0523.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–3. https://doi.org/10.1053/j.gastro.2020.02.055.
Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40(9):2095–103. https://doi.org/10.1111/liv.14455.
Liu W, Han R, Wu H, Han D. Viral threat to male fertility. Andrologia. 2018;50(11):e13140. https://doi.org/10.1111/and.13140.
Fan C, Lu W, Li K, Ding Y, Wang J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Infection in COVID-19 Patients. Front Med (Lausanne). 2020;7:563893. https://doi.org/10.3389/fmed.2020.563893.
Shen Q, Xiao X, Aierken A, Yue W, Wu X, Liao M, Hua J. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med. 2020;24(16):9472–7. https://doi.org/10.1111/jcmm.15541.
Aitken RJ. COVID-19 and male infertility: An update. Andrology. 2022;10(1):8–10. https://doi.org/10.1111/andr.13098.
Bao J, Guo Z, He J, Leng T, Wei Z, Wang C, Chen F. Semen parameters and sex hormones as affected by SARS-CoV-2 infection: A systematic review. Progrès en Urologie. 2022; https://doi.org/10.1016/j.purol.2022.09.004.
Koc E, Keseroglu BB. Does COVID-19 Worsen the Semen Parameters? Early Results of a Tertiary Healthcare Center. Urol Int. 2021;105(9–10):743–8. https://doi.org/10.1159/000517276.
Hamarat MB, Ozkent MS, Yilmaz B, Aksanyar SY, Karabacak K. Effect of SARS-CoV-2 infection on semen parameters. Can Urol Assoc J. 2022;16(3):E173-7. https://doi.org/10.5489/cuaj.7292.
Pazir Y, Eroglu T, Kose A, Bulut TB, Genc C, Kadihasanoglu M. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: A prospective cohort study. Andrologia. 2021;53(9):e14157. https://doi.org/10.1111/and.14157
Erbay G, Sanli A, Turel H, Yavuz U, Erdogan A, Karabakan M, Yaris M, Gultekin MH. Short-term effects of COVID-19 on semen parameters: A multicenter study of 69 cases. Andrology. 2021;9(4):1060–5. https://doi.org/10.1111/andr.13019.
Gul A, Zengin S, Dundar G, Ozturk M. Do SARS-CoV-2 Infection (COVID-19) and the Medications Administered for Its Treatment Impair Testicular Functions? Urol Int. 2021;105(11–12):944–8. https://doi.org/10.1159/000517925.
Chen F, Zhu S, Dai Z, Hao L, Luan C, Guo Q, Meng C, Zhang Y. Effects of COVID-19 and mRNA vaccines on human fertility. Hum Reprod. 2022;37(1):5–13. https://doi.org/10.1093/humrep/deab238.
Gonzalez DC, Khodamoradi K, Pai R, Guarch K, Connelly ZM, Ibrahim E, Arora H, Ramasamy R. A Systematic Review on the Investigation of SARS-CoV-2 in Semen. Res Rep Urol. 2020;12:615–21. https://doi.org/10.2147/RRU.S277679.
Zhang JJY, Lee KS, Ang LW, Leo YS, Young BE. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin Infect Dis. 2020;71(16):2199–206. https://doi.org/10.1093/cid/ciaa576.
Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, Li F, Xu Q, Zhang Y, Xu S, Song Z, Zeng Y, Shen Y, Shi Y, Zhu T, Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–6. https://doi.org/10.1016/j.jinf.2020.03.004.
Choi WS, Kang CI, Kim Y, Choi JP, Joh JS, Shin HS, Kim G, Peck KR, Chung DR, Kim HO, Song SH, Kim YR, Sohn KM, Jung Y, Bang JH, Kim NJ, Lee KS, Jeong HW, Rhee JY, et al. Clinical Presentation and Outcomes of Middle East Respiratory Syndrome in the Republic of Korea. Infect Chemother. 2016;48(2):118–26. https://doi.org/10.3947/ic.2016.48.2.118.
Lau SK, Woo PC, Yip CC, Tse H, Tsoi HW, Cheng VC, Lee P, Tang BS, Cheung CH, Lee RA, So LY, Lau YL, Chan KH, Yuen KY. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44(6):2063–71. https://doi.org/10.1128/JCM.02614-05.
Meng T, Dong R, Li T. Relationship between COVID-19 and the male reproductive system. Eur Rev Med Pharmacol Sci. 2021;25(2):1109–13.
Jannes P, Spiessens C, Van der Auwera I, D'Hooghe T, Verhoeven G, Vanderschueren D. Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice. Hum Reprod. 1998;13(2):372–5. https://doi.org/10.1093/humrep/13.2.372.
Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001;65(1):229–39. https://doi.org/10.1095/biolreprod65.1.229.
Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril. 2007;88(4):970.e1–7. https://doi.org/10.1016/j.fertnstert.2006.12.045.
Evenson DP, Jost LK, Corzett M, Balhorn R. Characteristics of human sperm chromatin structure following an episode of influenza and high fever: a case study. J Androl. 2000;21(5):739–46. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/10975421
Setchell B, Ekpe G, Zupp J, Surani M. Transient retardation in embryo growth in normal female mice made pregnant by males whose testes had been heated. Hum Reprod. 1998;13(2):342–7. https://doi.org/10.1093/humrep/13.2.342.
Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, Alkurt G, Doganay L, Yuruk E, Muslumanoglu AY. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: A cross-sectional, pilot study. Andrologia. 2021;53(2):e13912. https://doi.org/10.1111/and.13912.
Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, Kruessel JS, Bielfeld AP. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114(2):233–8. https://doi.org/10.1016/j.fertnstert.2020.05.028.
Donders GGG, Bosmans E, Reumers J, Donders F, Jonckheere J, Salembier G, Stern N, Jacquemyn Y, Ombelet W, Depuydt CE. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril. 2022;117(2):287–96. https://doi.org/10.1016/j.fertnstert.2021.10.022.
O'Donnell L, Stanton P, de Kretser DM. Endocrinology of the Male Reproductive System and Spermatogenesis. In: Feingold KR, et al., editors. Endotext. South Dartmouth (MA); 2000.
Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K, Zhou L, Shen S, Liu L, Liu Q, Xiong C. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. https://doi.org/10.1016/j.eclinm.2020.100604.
Yang M, Chen S, Huang B, Zhong JM, Su H, Chen YJ, Cao Q, Ma L, He J, Li XF, Li X, Zhou JJ, Fan J, Luo DJ, Chang XN, Arkun K, Zhou M, Nie X. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. Eur Urol Focus. 2020;6(5):1124–9. https://doi.org/10.1016/j.euf.2020.05.009.
Banihani SA. Human semen quality as affected by SARS-CoV-2 infection: An up-to-date review. Andrologia. 2022;54(2):e14295. https://doi.org/10.1111/and.14295.
Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol. 2003;58(1):1–26. https://doi.org/10.1016/s0165-0378(02)00060-8.
Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76(2):241–51. https://doi.org/10.1016/0092-8674(94)90332-8.
Orava M, Cantell K, Vihko R. Human leukocyte interferon inhibits human chorionic gonadotropin stimulated testosterone production by porcine Leydig cells in culture. Biochem Biophys Res Commun. 1985;127(3):809–15. https://doi.org/10.1016/s0006-291x(85)80015-2.
Tripathy AS, Vishwakarma S, Trimbake D, Gurav YK, Potdar VA, Mokashi ND, Patsute SD, Kaushal H, Choudhary ML, Tilekar BN, Sarje P, Dange VS, Abraham P. Pro-inflammatory CXCL-10, TNF-alpha, IL-1beta, and IL-6: biomarkers of SARS-CoV-2 infection. Arch Virol. 2021;166(12):3301–10. https://doi.org/10.1007/s00705-021-05247-z.
Chen Y, Wang J, Zhang Q, Xiang Z, Li D, Han X. Microcystin-leucine arginine exhibits immunomodulatory roles in testicular cells resulting in orchitis. Environ Pollut. 2017;229:964–75. https://doi.org/10.1016/j.envpol.2017.07.081.
Carneiro F, Teixeira TA, Bernardes FS, Pereira MS, Milani G, Duarte-Neto AN, Kallas EG, Saldiva PHN, Chammas MC, Hallak J. Radiological patterns of incidental epididymitis in mild-to-moderate COVID-19 patients revealed by colour Doppler ultrasound. Andrologia. 2021;53(4):e13973. https://doi.org/10.1111/and.13973.
Chen L, Huang X, Yi Z, Deng Q, Jiang N, Feng C, Zhou Q, Sun B, Chen W, Guo R. Ultrasound Imaging Findings of Acute Testicular Infection in Patients With Coronavirus Disease 2019: A Single-Center-Based Study in Wuhan, China. J Ultrasound Med. 2021;40(9):1787–94. https://doi.org/10.1002/jum.15558.
Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, Spivak AM, Alukal JP, Zhang X, Xiong C, Li PS, Hotaling JM. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135–9. https://doi.org/10.1016/j.fertnstert.2020.04.024.
Alkhatatbeh H, Alzaghari D, Alkhashman A, Azab M, Edwan GMA, Abufaraj M. Does severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) cause orchitis in patients with coronavirus disease 2019 (COVID-19)? Arab J Urol. 2020;18(3):129–33. https://doi.org/10.1080/2090598X.2020.1798862.
Ediz C, Tavukcu HH, Akan S, Kizilkan YE, Alcin A, Oz K, Yilmaz O. Is there any association of COVID-19 with testicular pain and epididymo-orchitis? Int J Clin Pract. 2021;75(3):e13753. https://doi.org/10.1111/ijcp.13753.
Okcelik S. COVID-19 pneumonia causes lower testosterone levels. Andrologia. 2021;53(1):e13909. https://doi.org/10.1111/and.13909.
J. Ning, W. Li, Y. Ruan, Y. Xia, X. Wu, K. Hu, X. Ding, X. Wu, L. Yu, and J. Zhou, "Effects of 2019 novel coronavirus on male reproductive system: a retrospective study," 2020.
Guo TH, Sang MY, Bai S, Ma H, Wan YY, Jiang XH, Zhang YW, Xu B, Chen H, Zheng XY, Luo SH, Xie XF, Gong CJ, Weng JP, Shi QH. Semen parameters in men recovered from COVID-19. Asian J Androl. 2021;23(5):479–83. https://doi.org/10.4103/aja.aja_31_21.
Mohammed N, Kamel M, Gadelkareem RA, Zarzour MA, Kurkar A, Abdel-Moniem AM, Behnsawy H. Semen quality changes during infection and recovery phases of mild-to-moderate COVID-19 in reproductive-aged patients: a prospective case series. Basic Clin Androl. 2023;33(1):2. https://doi.org/10.1186/s12610-022-00175-7.
Guo L, Zhao S, Li W, Wang Y, Li L, Jiang S, Ren W, Yuan Q, Zhang F, Kong F, Lei J, Yuan M. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2021;9(1):42–7. https://doi.org/10.1111/andr.12848.
Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, Xiong Y, Sun H, Zheng F, Chen Z, Qin J, Lyu J, Zhang Y, Zhang M. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93(1):456–62. https://doi.org/10.1002/jmv.26259.
Verma S, Saksena S, Sadri-Ardekani H. ACE2 receptor expression in testes: implications in coronavirus disease 2019 pathogenesisdagger. Biol Reprod. 2020;103(3):449–51. https://doi.org/10.1093/biolre/ioaa080.
Schonrich G, Raftery MJ, Samstag Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv Biol Regul. 2020;77:100741. https://doi.org/10.1016/j.jbior.2020.100741.
Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017;34(1):48–57. https://doi.org/10.1016/j.rbmo.2016.10.008.
Veenith T, Martin H, Le Breuilly M, Whitehouse T, Gao-Smith F, Duggal N, Lord JM, Mian R, Sarphie D, Moss P. High generation of reactive oxygen species from neutrophils in patients with severe COVID-19. Sci Rep. 2022;12(1):10484. https://doi.org/10.1038/s41598-022-13825-7.
Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J Urol. 2019;17(2):87–97. https://doi.org/10.1080/2090598X.2019.1599624.
Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79(4):829–43. https://doi.org/10.1016/s0015-0282(02)04948-8.
Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8(7):851–62. https://doi.org/10.2174/0929867013373039.
Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:12. https://doi.org/10.1186/1477-7827-2-12.
Pincemail J, Cavalier E, Charlier C, Cheramy-Bien JP, Brevers E, Courtois A, Fadeur M, Meziane S, Goff CL, Misset B, Albert A, Defraigne JO, Rousseau AF. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants (Basel). 2021;10(2) https://doi.org/10.3390/antiox10020257.
Alahmar AT. Role of Oxidative Stress in Male Infertility: An Updated Review. J Hum Reprod Sci. 2019;12(1):4–18. https://doi.org/10.4103/jhrs.JHRS_150_18.
Sawyer DE, Mercer BG, Wiklendt AM, Aitken RJ. Quantitative analysis of gene-specific DNA damage in human spermatozoa. Mutat Res. 2003;529(1-2):21–34. https://doi.org/10.1016/s0027-5107(03)00101-5.
Wang Z, Rhee DB, Lu J, Bohr CT, Zhou F, Vallabhaneni H, de Souza-Pinto NC, Liu Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010;6(5):e1000951. https://doi.org/10.1371/journal.pgen.1000951.
McAuley-Hecht KE, Leonard GA, Gibson NJ, Thomson JB, Watson WP, Hunter WN, Brown T. Crystal structure of a DNA duplex containing 8-hydroxydeoxyguanine-adenine base pairs. Biochemistry. 1994;33(34):10266–70. https://doi.org/10.1021/bi00200a006.
Wang D, Kreutzer DA, Essigmann JM. Mutagenicity and repair of oxidative DNA damage: insights from studies using defined lesions. Mutat Res. 1998;400(1-2):99–115. https://doi.org/10.1016/s0027-5107(98)00066-9.
De Iuliis GN, Thomson LK, Mitchell LA, Finnie JM, Koppers AJ, Hedges A, Nixon B, Aitken RJ. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biol Reprod. 2009;81(3):517–24. https://doi.org/10.1095/biolreprod.109.076836.
Shamsi MB, Kumar R, Bhatt A, Bamezai RN, Kumar R, Gupta NP, Das TK, Dada R. Mitochondrial DNA Mutations in etiopathogenesis of male infertility. Indian J Urol. 2008;24(2):150–4. https://doi.org/10.4103/0970-1591.40606.
Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14(8):470–85. https://doi.org/10.1038/nrurol.2017.69.
Panner Selvam MK, Sengupta P, Agarwal A. Sperm DNA fragmentation and male infertility. In: Genetics of male infertility. Springer; 2020. p. 155–72.
Falahieh FM, Zarabadipour M, Mirani M, Abdiyan M, Dinparvar M, Alizadeh H, Paktinat S, Hosseinirad H. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod Fertil Dev. 2021;33(12):683–90. https://doi.org/10.1071/RD21153.
Acknowledgments
None.
Funding
This work was supported by the Natural Science Foundation of Shandong Province [Grant No. ZR2020QC100] and Key Research and Development Project of Jining [Grant No. 2019SMNS018].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leng, T., Guo, Z., Sang, Z. et al. Effect of COVID-19 on sperm parameters: pathologic alterations and underlying mechanisms. J Assist Reprod Genet 40, 1623–1629 (2023). https://doi.org/10.1007/s10815-023-02795-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02795-y