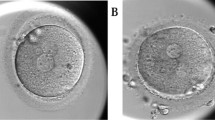
Abstract
Purpose
During fertilisation, female and male pronuclei (PNs) migrate to the centre of the ooplasm, juxtapose, and break down synchronously in preparation for the first mitosis. While PN non-juxtaposition and PN breakdown (PNBD) asynchrony are occasionally observed, their developmental implications remain uncertain. This study investigated the possible relationships among the two phenomena, preimplantation development patterns, and live birth rates in single blastocyst transfers.
Methods
A total of 1455 fertilised oocytes cultured in a time-lapse incubator were retrospectively analysed. Fertilised oocytes were divided into four groups according to the presence of PN juxtaposition and breakdown synchrony. The relationships of abnormal PN behaviour with embryo morphokinetics, blastocyst formation, and live birth were evaluated.
Results
PN non-juxtaposition and asynchrony were observed in 1.9% and 1.0% of fertilised oocytes, respectively. The blastocyst cryopreservation rates in the synchronous–non-juxtaposed and asynchronous–non-juxtaposed groups were significantly lower than that in the synchronous–juxtaposed group. The rates of clinical pregnancy, ongoing pregnancy, and live birth were comparable among the groups. Non-juxtaposition was significantly associated with increased trichotomous cleavage at the first cytokinesis (P < 0.0001) and an increase in the time interval from PNBD to first cleavage (P < 0.0001). Furthermore, asynchronous PNBD was significantly correlated with increased rapid cleavage at the first cytokinesis (P = 0.0100).
Conclusion
Non-juxtaposition and asynchronous PNBD is associated with abnormal mitosis at the first cleavage and impaired preimplantation development. However, embryos displaying abnormal PNBD may develop to blastocyst stage and produce live births, suggesting blastocyst transfer as a more appropriate culture strategy.

Similar content being viewed by others
Abbreviations
- AJ:
-
asynchronous–juxtaposed
- AN:
-
asynchronous–non-juxtaposed
- CC:
-
clomiphene citrate
- FSH:
-
follicle-stimulating hormone
- hMG:
-
human menopausal gonadotropin
- HTF:
-
human tubal fluid
- ICM:
-
inner cell mass
- ICSI:
-
intracytoplasmic sperm injection
- NPB:
-
nucleolus precursor body
- PN:
-
pronuclei
- PNBD:
-
pronuclei breakdown
- rFSH:
-
recombinant follicle-stimulating hormone
- SJ:
-
synchronous–juxtaposed
- SN:
-
synchronous–non-juxtaposed
- SVBT:
-
single vitrified-warmed blastocyst transfer
- t2:
-
two-cell stage
- t3:
-
three-cell stage
- TE:
-
trophectoderm
- tHa:
-
time of halo initiation
- tHd:
-
time at which the halo phenomenon disappeared
- tHr:
-
time at which the granules started to redistribute
- TI:
-
time interval
- tPB2:
-
second polar body
- tPN1:
-
the appearance of the first PN
- tPN2:
-
the appearance of the second PN
- tPNf:
-
pronuclei fading
References
Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nat Cell Biol. 2001;3:E59–64.
Evans JP. Sperm-egg interaction. Annu Rev Physiol. 2012;74:477–502.
Klinovska K, Sebkova N, Dvorakova-Hortova K. Sperm-egg fusion: a molecular enigma of mammalian reproduction. Int J Mol Sci. 2014;15:10652–68.
Sutovsky P. Sperm-egg adhesion and fusion in mammals. Expert Rev Mol Med. 2009;11:e11.
Coticchio G, Mignini Renzini M, Novara PV, Lain M, De Ponti E, Turchi D, Fadini R, Dal CM. Focused time-lapse analysis reveals novel aspects of human fertilization and suggests new parameters of embryo viability. Hum Reprod. 2018;33:23–31.
Liu Y, Chapple V, Roberts P, Ali J, Matson P. Time-lapse videography of human oocytes following intracytoplasmic sperm injection: events up to the first cleavage division. Reprod Biol. 2014;14:249–56.
Aguilar J, Motato Y, Escriba MJ, Ojeda M, Munoz E, Meseguer M. The human first cell cycle: impact on implantation. Reprod BioMed Online. 2014;28:475–84.
Azzarello A, Hoest T, Mikkelsen AL. The impact of pronuclei morphology and dynamicity on live birth outcome after time-lapse culture. Hum Reprod. 2012;27:2649–57.
Kirkegaard K, Kesmodel US, Hindkjaer JJ, Ingerslev HJ. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod. 2013;28:2643–51.
Apter S, Ebner T, Freour T, Guns Y, Kovacic B, Le Clef N, Marques M, Meseguer M, Montjean D, Sfontouris I, Sturmey R, Coticchio G. Good practice recommendations for the use of time-lapse technology (dagger). Hum Reprod Open. 2020;2020:hoaa008.
Athayde Wirka K, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, Suraj V, Tan L, Shen S. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101:1637–1648 e1-5.
Ezoe K, Hickman C, Miki T, Okimura T, Uchiyama K, Yabuuchi A, Kobayashi T, Coticchio G, Kato K. Cytoplasmic halo characteristics during fertilization and their implications for human preimplantation embryo development and pregnancy outcome. Reprod BioMed Online. 2020;41:191–202.
Gianaroli L, Magli MC, Ferraretti AP, Fortini D, Grieco N. Pronuclear morphology and chromosomal abnormalities as scoring criteria for embryo selection. Fertil Steril. 2003;80:341–9.
Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, Cutting R, Ong K, Sallam H, Li TC. Recurrent implantation failure: definition and management. Reprod BioMed Online. 2014;28:14–38.
Kato K, Ezoe K, Yabuuchi A, Fukuda J, Kuroda T, Ueno S, Fujita H, Kobayashi T. Comparison of pregnancy outcomes following fresh and electively frozen single blastocyst transfer in natural cycle and clomiphene-stimulated IVF cycles. Hum Reprod Open. 2018;3:hoy006.
Ezoe K, Ni X, Kobayashi T, Kato K. Anti-Mullerian hormone is correlated with cumulative live birth in minimal ovarian stimulation with clomiphene citrate: a retrospective cohort study. BMC Preg Childbirth. 2020;20:740.
Karakida S, Ezoe K, Fukuda J, Yabuuchi A, Kobayashi T, Kato K. Effects of gonadotropin administration on clinical outcomes in clomiphene citrate-based minimal stimulation cycle IVF. Reprod Med Biol. 2020;19:128–34.
Kato K, Takehara Y, Segawa T, Kawachiya S, Okuno T, Kobayashi T, Bodri D, Kato O. Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-centre, Japanese cohort. Reprod Biol Endocrinol. 2012;10:35.
Teramoto S, Kato O. Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study. Reprod BioMed Online. 2007;15:134–48.
Mori C, Yabuuchi A, Ezoe K, Murata N, Takayama Y, Okimura T, Uchiyama K, Takakura K, Abe H, Wada K, Okuno T, Kobayashi T, Kato K. Hydroxypropyl cellulose as an option for supplementation of cryoprotectant solutions for embryo vitrification in human assisted reproductive technologies. Reprod BioMed Online. 2015;30:613–21.
Ezoe K, Ohata K, Morita H, Ueno S, Miki T, Okimura T, Uchiyama K, Yabuuchi A, Kobayashi T, Montag M, Kato K. Prolonged blastomere movement induced by the delay of pronuclear fading and first cell division adversely affects pregnancy outcomes after fresh embryo transfer on Day 2: a time-lapse study. Reprod BioMed Online. 2019;38:659–68.
Coticchio G, Ezoe K, Lagalla C, Shimazaki K, Ohata K, Ninomiya M, Wakabayashi N, Okimura T, Uchiyama K, Kato K, Borini A. Perturbations of morphogenesis at the compaction stage affect blastocyst implantation and live birth rates. Hum Reprod. 2021;36:918–28.
Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage patterns and subsequent karyotypic analysis of embryos. Biol Reprod. 1987;37:395–401.
Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, Bellver J, Meseguer M. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–63.
Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, Sayed S. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29:2650–60.
Veeck LL. Abnormal morphology of human oocytes and conceptus. Atlas of the human oocyte and early conceptus. 2nd ed. Abnormal morphology of human oocytes and conceptus. Baltimore: Williams & Wilkins; 1996.
Gardner D, Schoolcraft W. In vitro culture of human blastocysts. Towards Reproductive Certainty: Fertility and Genetics Beyond. New York: Parthenon Publishing Carnforth; 1999.
Clift D, Schuh M. Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 2013;14:549–62.
Gonczy P, Pichler S, Kirkham M, Hyman AA. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol. 1999;147:135–50.
Kim NH, Simerly C, Funahashi H, Schatten G, Day BN. Microtubule organization in porcine oocytes during fertilization and parthenogenesis. Biol Reprod. 1996;54:1397–404.
Payne C, Rawe V, Ramalho-Santos J, Simerly C, Schatten G. Preferentially localized dynein and perinuclear dynactin associate with nuclear pore complex proteins to mediate genomic union during mammalian fertilization. J Cell Sci. 2003;116:4727–38.
Cavazza T, Takeda Y, Politi AZ, Aushev M, Aldag P, Baker C, Choudhary M, Bucevicius J, Lukinavicius G, Elder K, Blayney M, Lucas-Hahn A, Niemann H, Herbert M, Schuh M. Parental genome unification is highly error-prone in mammalian embryos. Cell. 2021;184(11):2860–77.
Scott L. Pronuclear scoring as a predictor of embryo development. Reprod BioMed Online. 2003;6:201–14.
Fishman EL, Jo K, Nguyen QPH, Kong D, Royfman R, Cekic AR, Khanal S, Miller AL, Simerly C, Schatten G, Loncarek J, Mennella V, Avidor-Reiss T. A novel atypical sperm centriole is functional during human fertilization. Nat Commun. 2018;9:2210.
Faramarzi A, Khalili MA, Omidi M, Agha-Rahimi A, Taheri F. Pronuclear pattern does not predict morphokinetics behavior in human embryos. Gynecol Endocrinol. 2018;34:248–51.
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, Reijo Pera RA. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28:1115–21.
Availability of data and material
The primary data for this study are available from the authors on direct request.
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Kenji Ezoe designed the study; Kenji Ezoe, Hitomi Takenouchi, Shota Taoda, Shima Namerikawa, Kasumi Honda, and Tetsuya Miki observed and annotated embryo development; Kenji Ezoe analysed the data; Kenji Ezoe, Giovanni Coticchio, and Keiichi Kato wrote the paper; Tadashi Okimura, Tamotsu Kobayashi, and Andrea Borini revised the paper.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Review Board of the Kato Ladies Clinic (approval number: 16-32).
Consent to participate
Written informed consent for the retrospective analysis of de-identified data was obtained from patients undergoing in vitro fertilisation treatment at the centre.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Fertilised oocyte that exhibited pronuclei non-juxtaposition and synchronous pronuclei breakdown (WMV 12917 kb)
Fertilised oocyte that exhibited pronuclei juxtaposition and asynchronous pronuclei breakdown (breakdown) (WMV 14147 kb)
Fertilised oocyte that exhibited pronuclei juxtaposition and asynchronous pronuclei breakdown (shrinkage) (WMV 13009 kb)
Fertilised oocyte that exhibited pronuclei non-juxtaposition and asynchronous pronuclei breakdown (WMV 14735 kb)
ESM 5
Supplementary information Table 1 Embryonic outcomes stratified by the patient and characteristics of pronuclei behaviour. Supplementary information Table 2 Multivariate logistic regression analysis for pregnancy outcomes in embryos with anomalies in pronuclei breakdown. Supplementary information Table 3 Embryonic outcomes of fertilised oocytes with non-juxtaposition, stratified by the distance between two pronuclei. Supplementary information Table 4 Embryonic outcomes of fertilised oocytes with asynchronous pronuclei breakdown, stratified by the time intervals of pronuclei breakdown asynchrony. (DOCX 48 kb)

Fig. S1
Flowchart describing the method of patient selection, including inclusion and exclusion criteria. Women who previously underwent embryo transfer four or more times were defined as experiencing recurrent implantation failure (PNG 136 kb)
Rights and permissions
About this article
Cite this article
Ezoe, K., Coticchio, G., Takenouchi, H. et al. Spatiotemporal perturbations of pronuclear breakdown preceding syngamy affect early human embryo development: a retrospective observational study. J Assist Reprod Genet 39, 75–84 (2022). https://doi.org/10.1007/s10815-021-02335-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02335-6