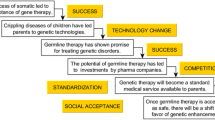
Abstract
Science, propelled forward by noble aspirations and, at times, human hubris, has the capacity to affect lives and alter the world in unanticipated ways. Even seemingly minor discoveries have repeatedly proven to have far reaching implications that experts within their respective fields could not have predicted. Nuclear technology is both a source of energy and a potential means of annihilation. The internet has both seamlessly connected the world but has also opened society to the misuse and manipulation of information. Both exemplify how new technologies have the potential for positive and negative outcomes that often go beyond what was initially intended. This is not a fault of science and innovation but rather an inherent occupational hazard as new discoveries exist within a gray zone between ignorance and comprehension. These gaps in our knowledge can only be filled over time as our knowledge expands. Innovations that were once seen as fringe, over time, become mainstream and that which was once revolutionary becomes a part of everyday life. Occasionally, a scientific advancement comes along that challenges societal norms and causes us to question what is feasible, acceptable, and ethical. Nowhere in the twenty-first century has this been more evident than within the fields of genetics and genetic engineering. As we gain a deeper understanding of the source code of life, from individual base pairs to epigenetic influences, the implications of new discoveries will go far beyond curing genetic diseases, and the possibilities will be endless. Reproductive endocrinology and infertility (REI) specialists utilize many tools including expanded carrier screening, preimplantation genetic testing, and embryo selection and have become some of the experts at the forefront of the ongoing genetic revolution. Now more than ever, there is a need for REIs to be trained in the fundamentals of genetics, exposed to novel gene sequencing and editing techniques, and involved in the coming ethical discussions in order to be prepared for the genetically engineered future.
Similar content being viewed by others
References
Chinese researcher claims birth of first gene-edited babies - STAT [Internet]. Available from: https://www.statnews.com/2018/11/25/china-first-gene-edited-babies-born/. Accessed 4/9/2020.
“CRISPR babies” scientist He Jiankui rose from obscurity to stun the world [Internet]. Available from: https://www.statnews.com/2018/12/17/crispr-shocker-genome-editing-scientist-he-jiankui/. Accessed 4/9/2020.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21.
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154(2):442–51.
Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. eLife. 2013;2:e00471.
Zhou M, Greenhill S, Huang S, Silva TK, Sano Y, Wu S, et al. CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory. eLife. 2016;5:e20985.
Jones K, Maguire J, Davenport A. Chemokine receptor CCR5: from AIDS to atherosclerosis: CCR5: from AIDS to atherosclerosis. Br J Pharmacol. 2011;162(7):1453–69.
Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203(1):35–40.
Falcon A, Cuevas MT, Rodriguez-Frandsen A, Reyes N, Pozo F, Moreno S, et al. CCR5 deficiency predisposes to fatal outcome in influenza virus infection. J Gen Virol. 2015;96(8):2074–8.
Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
Jeggo PA. 5 DNA Breakage and Repair [Internet]. In: Advances in Genetics. Elsevier; 1998. p. 185–218.Available from: https://linkinghub.elsevier.com/retrieve/pii/S0065266008601443. Accessed 4/13/2020.
Antonarakis SE, Beckmann JS. Mendelian disorders deserve more attention. Nat Rev Genet. 2006;7(4):277–82.
Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao S, et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell. 2013;13(6):659–62.
Long C, Li H, Tiburcy M, Rodriguez-Caycedo C, Kyrychenko V, Zhou H, et al. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci Adv. 2018;4(1):eaap9004.
Ma H, Marti-Gutierrez N, Park S-W, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413–9.
Metzl J. Hacking Darwin: genetic engineering and the future of humanity. Naperville: Sourcebooks, Inc; 2019.
Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–5.
Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504(7479):282–6.
Irie N, Surani MA. Efficient induction and isolation of human primordial germ cell-like cells from competent human pluripotent stem cells. In: Buszczak M, editor. Germline Stem Cells. New York: Springer New York; 2017 [cited 2020 Apr 15]. p. 217–26.Available from: https://doi.org/10.1007/978-1-4939-4017-2_16
Irie N, Kim S, Surani MA. Human germline development from pluripotent stem cells in vitro. J Mamm Ova Res 2016;33(2):79–87.
Lewin T. Coming to U.S. for Baby, and Womb to Carry It [Internet]. N. Y. Times. 2014;Available from: https://www.nytimes.com/2014/07/06/us/foreign-couples-heading-to-america-for-surrogate-pregnancies.html. Accessed 4/20/2020.
Lander ES, Baylis F, Zhang F, Charpentier E, Berg P, Bourgain C, et al. Adopt a moratorium on heritable genome editing. Nature. 2019;567(7747):165–8.
Baylis F. Altered inheritance: CRISPR and the ethics of human genome editing. Cambridge: Harvard University Press; 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pavlovic, Z.J., Sax, M.R., Kim, A.S. et al. Altered evolution: are reproductive endocrinology and infertility specialists ready for the genetically engineered future?. J Assist Reprod Genet 37, 2949–2954 (2020). https://doi.org/10.1007/s10815-020-01963-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01963-8