Abstract
Purpose
The aim of this study is to compare implantation and live birth rates (LBR) between fresh euploid embryo transfers versus cryo-all cycles with a subsequent embryo transfer into a prepared endometrium.
Material and Methods
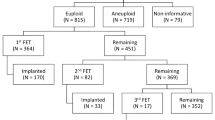
This is a retrospective cohort study. Patients who underwent an IVF cycle with PGS with trophectoderm biopsy from January 2011 to July 2015 were included. Patients were divided into three groups: “Fresh Only,” “Frozen Embryo Transfer ('FET) Only,” and “Fresh ET then FET.” For “Fresh Only” group (n = 345), PGS results were received within 24 h. For “FET Only” group (n = 514), results were expected after 24 h, and embryos were cryopreserved after biopsy; only FET was performed in this group (no fresh transfer). For “FET with a previous fresh ET” (n = 139) group, patients underwent a fresh ET with a subsequent FET, in which the same cohort of embryos was utilized. The main outcome measures were pregnancy rate (PR), clinical PR, implantation rate (IR), LBR, and early pregnancy loss rate.
Results
IRs were statistically higher in the “FET Only” group when compared to the “Fresh Only” group (59.5 vs. 50.6 %, p < 0.01) and the “FET with a previous fresh ET” (59.5 vs. 50.6 %, p < 0.05). LBR was statistically significant in the “FET Only” group when compared to the “Fresh Only” group (57.6 vs. 46.5 %, p < 0.005) but not when compared to “FET with a previous fresh ET” group (57.6 vs. 47.7 %, p = 0.07).
Conclusions
This analysis suggests euploid embryos to be more likely to implant and achieve a LBR in a synthetic FET cycle than in a fresh cycle.

Similar content being viewed by others
References
Fatemi HM, Popovic-Todorovic B. Implantation in assisted reproduction: a look at endometrial receptivity. Reprod Biomed Online. 2013;27:530–8.
Koot Y, Maklon N. Embryo implantation: biology, evaluation and enhancement. Curr Opin Obstet Gynecol. 2013;25(4):274–9.
Queenan Jr JT, Veeck LL, Seltman HJ, Muasher SJ. Transfer of cryopreserved-thawed pre-embryos in a natural cycle or a programmed cycle with exogenous hormonal replacement yields similar pregnancy results. Fertil Steril. 1994;62:545–50.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–8.
Levi AJ, Drews MR, Bergh PA, Miller BT, Scott Jr RT. Controlled ovarian hyperstimulation does not adversely affect endometrial receptivity in in vitro fertilization cycles. Fertil Steril. 2001;76:670–4.
von Grothusen C, Lalitkumar S, Rao Boggavarapu N, Gemzell-Danielsson K, Lalitkumar PG. Recent advances in understanding endometrial receptivity: molecular basis and clinical applications. Am J Reprod Immunol. 2014;72(2):148–57.
Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99(1):156–62.
Cobo A, Santos MJ d l, Castello D, Gamiz P, Campos P, Remohi J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150warming cycles. Fertil Steril. 2012;98:1138–46. e1131.
Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96(2):277–85.
Wilton L. Preimplantation genetic diagnosis for aneuploidy screening in early human embryos: a review. Prenat Diagn. 2002;22:512–8.
Hardy K, Martin K, Leese H, Winston R, Handyside A. Human preimplantation development in vitro is not adversely affected by biopsy at the 8-cell stage. Hum Reprod. 1990;5:708–14.
Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015;30(2):473–83.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80.
Fatemi HM, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. 2010;94(6):2054–8.
Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet. 2010;27(7):357–63.
Chang EM, Han JE, Won HJ, Kim YS, Yoon TK, Lee WS. Effect of estrogen priming through luteal phase and stimulation phase in poor responders in in-vitro fertilization. J Assist Reprod Genet. 2011;29:225–30.
El Bahja D, Hertz P, Schweitzer T, Lestrade F, Ragage JP. Frozen embryo transfer protocol: does spontaneous cycle give good results? Gynecol Obstet Fertil. 2013;41:648–52.
Xiao Z, Zhou X, Xu W, Yang J, Xie Q. Natural cycle is superior to hormone replacement therapy cycle for vitrificated-preserved frozen-thawed embryo transfer. Syst Biol Reprod Med. 2012;58:107–12.
Zhu D, Zhang J, Cao S, Zhang J, Chin Heng B, Huang M, et al. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles –time for a new embryo transfer strategy? Fertil Steril. 2011;95(5):1691–5.
Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340(23):1796–9.
Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14:236–42.
Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertil Steril. 2009;91:749–66.
Thomas K, Thomson AJ, Sephton V, Cowan C, Wood S, Vince G, et al. The effect of gonadotrophic stimulation on integrin expression in the endometrium. Hum Reprod. 2002;17:63–8.
Garrido-Gómez T1, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simón C. Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril. 2013;99(4):1078–85.
Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Embryo cryopreservation rescues cycles with premature luteinization. Fertil Steril. 2010;93:636–41.
Al-Azemi M, Kyrou D, Kolibianakis EM, Humaidan P, van Vaerenbergh I, Devroey P, et al. Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online. 2012;24:381–8.
Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update. 2007;13:343–55.
Franasiak J, Forman EJ, Hong KH, Werner MD, Upham KM, Scott RT. Investigating the impact of the timing of blastulation on implantation: active management of embryo-endometrial synchrony increases implantation rates. Fertil Steril. 2013;100(3):S97.
Navot D, Scott RT, Droesch K, Veeck LL, Liu HC, Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril. 1991;55:114–8.
Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25.
Blesa D, Ruiz-Alonso M, Simón C. Clinical management of endometrial receptivity. Semin Reprod Med. 2014;32(05):410–4.
Lessey BA, Castelbaum AJ, Sawin SW, SUN J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63(3):535–42.
Dubowy RL, Feinberg RF, Keefe DL, et al. Improved endometrial assessment using cyclin E and p27. Fertil Steril. 2003;80(1):146–56.
Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95(1):50–60. e1–e15.
Practice Committee of American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion. Fertil Steril. 2012;97(4):825–34.
Griesinger G, Berndt H, Schultz L, Depenbusch M, Schultze-Mosgau A. Cumulative live birth rates after GnRH-agonist triggering of final oocyte maturation in patients at risk of OHSS: a prospective, clinical cohort study. Eur J Obstet Gynecol Reprod Biol. 2010;149(2):190–4.
Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2003;80(5):1309–14.
Roque. Freeze-all policy: is it time for what? J Assist Reprod Genet. 2014 Nov 27.
Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in IVF cycles. Fertil Steril. 2006;85:112–20.
Lewitt N, Kol S, Manor D, Itskovitz-Eldor J. Comparison of gonadotrophin-releasing hormone analogues and human chorionic gonadotrophin for the induction of ovulation and prevention of ovarian hyperstimulation syndrome: a case–control study. Hum Reprod. 1996;11:1399–402.
Aytoz et al. Obstetric outcome of pregnancies after the transfer of cryopreserved and fresh embryos obtained by conventional in-vitro fertilization and intracytoplasmatic sperm injection. Human Reprod. 1999;14(10):2619–24.
Kansal Kalra S et al. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril. 2011;95(2):548–53.
Wennerholm UB et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Human Reprod. 2013;28(9):2545–53.
Maheshwari A et al. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98(2):368–377.e9.
Pelkonen S, Hartikainen AL, Ritvanen A, Koivunen R, Martikainen H, Gissler M, et al. Major congenital anomalies in children born after frozen embryo transfer: a cohort study 1995–2006. Hum Reprod. 2014;29(7):1552–7.
Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29(3):618–27.
Saravelos SH, Regan L. Unexplained recurrent pregnancy loss. Obstet Gynecol Clin North Am. 2014;41(1):157–66.
Christiansen OB, Nybo Andersen AM, Bosch E, Daya S, Delves PJ, Hviid TV, et al. Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril. 2005;83(4):821–39. Review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Capsule This analysis suggests euploid embryos to be more likely to implant and achieve a LBR in a synthetic FET cycle than in a fresh cycle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Demographic characteristics of Only IVF vs. Only FET. Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Only IVF set against the quantiles of the Only FET data set testing the normality of each group (DOCX 1029 kb)
ESM 2
Laboratory outcomes of Only IVF vs. Only FET. Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Only IVF set against the quantiles of the Only FET data set testing the normality of each group (DOCX 780 kb)
ESM 3
Demographic characteristics of Only FET vs. Fresh then FET. Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Only FET set against the quantiles of the Fresh then FET data set testing the normality of each group (DOCX 855 kb)
ESM 4
Laboratory outcomes of Only FET vs. Fresh then FET. Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Only FET set against the quantiles of the Fresh then FET data set testing the normality of each group (DOCX 756 kb)
ESM 5
Demographic characteristics of patient s in the Fresh then FET group segregated according if they fresh cycle was successful (pregnant vs. non-pregnant). Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Pregnant set against the quantiles of the Non-Pregnant data set testing the normality of each group (DOCX 412 kb)
ESM 6
Laboratory outcomes of patient s in the Fresh then FET group segregated according if they fresh cycle was successful (pregnant vs. non-pregnant). Histogram of the distribution with overlaid normal and kernel densities of all demographic variables. Boxplot comparing the distribution of each demographic variable. Q-q plot of the quantiles of the Pregnant set against the quantiles of the Non-Pregnant data set testing the normality of each group (DOCX 401 kb)
ESM 7
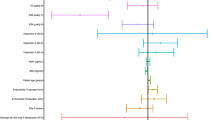
Comparison of patients in the ‘Fresh ET then FET’ segregated according if they fresh cycle was successful (pregnant vs. non-pregnant). Results are expressed as mean ± standard deviation with 95 % confidence intervals (CI). Significance established at p < 0.05. For each group, pregnancy rates, clinical pregnancy rates, early pregnancy loss rates, multiple pregnancy rates, and live birth rates were calculated. Adjusted odds ratios (OR) and their 95 % CI by Clopper-Pearson method for implantation rate, PR, clinical PR, and early pregnancy loss rate were calculated to evaluate the relative odds of each event compared with the reference group (Fresh Only) (DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Rodriguez-Purata, J., Lee, J., Whitehouse, M. et al. Reproductive outcome is optimized by genomic embryo screening, vitrification, and subsequent transfer into a prepared synchronous endometrium. J Assist Reprod Genet 33, 401–412 (2016). https://doi.org/10.1007/s10815-016-0647-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0647-y