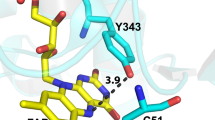
The nitration of tyrosyl residues in proteins is considered a type of post-translational modification of proteins, indicating disruptions in the metabolic and signaling functions of nitric oxide •NO and the development of oxidative nitrosative stress. We have examined the nonenzymatic pathway of protein nitration by the action of visible light in the presence of riboflavin and nitrite. Mass spectrometry was used to show that riboflavin-photosensitized redox processes involving nitrite and tyrosine or tyrosyl residues lead to nitration of residues Tyr-103 and Tyr-146 in the polypeptide chain of horse heart myoglobin. A possible role is discussed for riboflavin and other natural photosensitizers in the modification and damage of proteins when the body is exposed to intense visible light in the presence of nitrites in the blood.
Similar content being viewed by others
References
K. Chandramouli and P.-Y. Qian, Hum. Genom. Proteom., 2009, Article ID 239204 (2009).
Y. Zhang, B. R. Fonslow, B. Shan, M.C. Baek, and J. R. Yates III, Chem. Rev., 113, No. 4, 2343–2394 (2013).
O. N. Jensen, Nat. Rev. Mol. Cell Biol., 7, No. 6, 391–403 (2006).
S. Prabakaran, G. Lippens, H. Steen, and J. Gunawardena, Wiley Interdiscip. Rev. Syst. Biol. Med., 4, No. 6, 565–583 (2012).
G. A. Khoury, R. C. Baliban and C. A. Floudas, Sci. Rep., 1, No. 1, 1–5 (2011).
E. Gianazza, J. Crawford, and I. Miller, Amino Acids, 33, 51–56 (2007).
R. Radi, Proc. Natl. Acad. Sci. USA, 101, No. 12, 4003–4008 (2004).
R. Radi, Proc. Natl. Acad. Sci. USA, 115, No. 23, 5839–5848 (2018).
L. A. Abriata, A. Cassina, V. Tortora, M. Marin, J. M. Souza, L. Castro, A. J. Vila, and R. Radi, J. Biol. Chem., 284, No. 1, 17–26 (2009).
C. Szabó, H. Ischiropoulos, and R. Radi, Nat. Rev. Drug Disc., 6, No. 8, 662–680 (2007).
S. Herold, Free Radical Biol. Med., 36, No. 5, 565–579 (2004).
J. B. Sampson, Y. Ye, H. Rosen, and J. S. Beckman, Arch. Biochem. Biophys., 356, No. 2, 207–213 (1998).
K. Bian, Z. Gao, N. Weisbrodt, and F. Murad, Proc. Natl. Acad. Sci. USA, 100, No. 10, 5712–5717 (2003).
A. van der Vliet, J. P. Eiserich, B. Halliwell, and C. E. Cross, J. Biol. Chem., 272, No. 12, 7617–7625 (1997).
J. P. Eiserich, M. Hristova, C. E. Cross, A. D. Jones, B. A. Freeman, B. Halliwell, and A. van der Vliet, Nature, 391, No. 6665, 393–397 (1998).
K. Kiline, A. Kiline, R. E. Wolf, and M. B. Grisham, Biochem. Biophys. Res. Commun., 285, No. 2, 273–276 (2001).
I. Stepuro, A. V. Oparin, V. Stsiapura, S. Maskevich, and V. V. Titov, Biochemistry (Moscow), 77, No. 1, 41–55 (2012).
S. Labor, V. Stepuro, I. Stepuro, and V. Smirnov, Izv. Nats. Akad. Nauk Belarusi, Ser. Biol. Nauk., No. 2, 55–65 (2017).
T. Spolitak, P. F. Hollenberg, and D. P. Ballou, Arch. Biochem. Biophys., 600, 33–46 (2016).
S. Nicolis, A. Pennati, R. Ronconi, L. Gianelli, and L. Casella, Chem. Eur. J., 10, No. 9, 2281–2290 (2004).
S. Nicolis, A. Pennati, E. Perani, E. Monzani, A. M. Sanangelantoni, and L. Casella, Chem. Eur. J., 12, No. 3, 749–757 (2006).
K. Shikama and Y. Sugawara, Eur. J. Biochem., 91, No. 2, 407–413 (1978).
Y. Sugawara and K. Shikama, Eur. J. Biochem., 110, No. 1, 241–246 (1980).
G.I. Tajima and K. Shikama, J. Biol. Chem., 262, No. 26, 12603–12606 (1987).
M. R. Gunther, V. Sampath, and W. S. Caughey, Free Radical Biol. Med., 26, Nos. 11–12, 1388–1395 (1999).
I. Stepuro and V. Stepuro, Oxidation of Thiamine Derivatives [in Russian], Lambert Academic Publishing (2014).
H. Ischiropoulos, Arch. Biochem. Biophys., 356, No. 1, 1–11 (1998).
M. S. Baptista, J. Cadet, A. Greer, and A. H. Thomas, Photochem. Photobiol., 97, No. 6, 1456–1483 (2021).
M. S. Baptista, J. Cadet, P. Di Mascio, A. A. Gbogare, A. Greer, M. R. Hamblin, C. Lorente, S. C. Nunez, M. S. Ribeiro, and A. H. Thomas, Photochem. Photobiol., 93, No. 4, 912–919 (2017).
F. Wilkinson, W. P. Helman, and A. B. Ross, J. Phys. Chem. Ref. Data, 22, No. 1, 113–262 (1993).
C. S. Foote, in: W. Pryor (Ed.), Free Radicals in Biology, Academic Press, New York (2976), pp. 85–133.
A. M. Edwards and E. Silva, J. Photochem. Photobiol. B: Biol., 63, No. 1, 126–131 (2001).
M. Sinsińska-Rak and M. Sikorski, Chem. Eur. J., 20, No. 47, 15280–15291 (2014).
T. Sinha, M. I. Naash, and M. R. Al-Ubaidi, Front. Cell Dev. Biol., 8, 861 (2020).
D. R. Cardoso, S. H. Libardi, and L. H. Skibsted, Food Funct., 3, No. 5, 487–502 (2012).
C. Y. Lu and Y. Y. Liu, Biochim. Biophys. Acta Gen. Subj., 1571, No. 1, 71–76 (2002).
H. Ostdal, B. Daneshvar, and L. H. Skibsted, Free Radical Res., 24, No. 6, 429–438 (1996).
G. S. Bayse, A. W. Michaels, and M. Morrison, Biochim. Biophys. Acta, Enzymol. Biol. Oxid., 284, No. 1, 34–42 (1972).
S. O. Anderson, Acta Physiol. Scand. Suppl., 263, 1–81 (1966).
I. Stepuro, Prostaglandins Leukot. Essent. Fatty Acids, 72, No. 2, 115–127 (2005).
E. Antonini and M. Brunori, Hemoglobin and Myoglobin in Their Reactions with Ligands, North-Holland Publishing Company (1971).
C. W. Fenwick, A. M. English, and J. F. Wishart, J. Am. Chem. Soc., 119, No. 20, 4758–4764 (1997).
W. H. Koppenol, D. M. Stanbury, and P. L. Bounds, Free Rad. Biol. Med., 49, No. 3, 317–322 (2010).
D. M. Stanbury, in: A. G. Sykes (Ed.), Advanced Inorganic Chemistry, Academic Press, New York (1989), pp. 69–138.
M. D. Bartberger, W. Liu, E. Ford, K. M. Miranda, C. Switzer, J. M. Fukuto, P. J. Farmer, D. A. Wink, and K. N. Houk, Proc. Natl. Acad. Sci. USA, 99, No. 17, 10958–10964 (2002).
A. Harriman, J. Phys. Chem., 91, No. 24, 6102–6102 (1987).
D. A. Malencik and S. R. Anderson, Amino Acids, 25, No. 3, 233–247 (2003).
A. Wright, W. A. Bubb, C. L. Hawkins, and M. J. Davies, Photochem. Photobiol., 76, No. 1, 35–46 (2002).
P. Nagy, A. J. Kettle, and C. C. Winterbourn, J. Biol. Chem., 284, No. 22, 14723–14733 (2009).
M. N. Möller, D. M. Hatch, H.Y. H. Kim, and N. A. Porter, J. Am, Chem. Soc., 134, No. 40, 16773–16780 (2012).
J. R. Harbour and S. L. Issler, J. Am. Chem. Soc., 104, No. 3, 903–905 (1982).
W. Koppenol, J. Moreno, W. A. Pryor, H. Ischiropoulos, and J. Beckman, Chem. Res. Toxicol., 5, No. 6, 834–842 (1992).
M. Fontana, C. Blarzino, and L. Pecci, Amino Acids, 42, No. 5, 1857–1865 (2012).
D. W. Batey and C. D. Eckhert, Anal. Biochem., 188, No. 1, 164–167 (1990).
A. M. Alperstein, J. S. Ostrander, T. O. Zhang, and M. T. Zanni, Proc. Natl. Acad. Sci. USA, 116, No. 14, 6602–6607 (2019).
G. Viteri, A. M. Edwards, J. De la Fuenta, and E. Silva, Photochem. Photobiol., 77, No. 5, 535–540 (2003).
E. L. Padgett and S. B. Pruett, Biochem. Biophys. Res. Commun., 186, No. 2, 775–781 (1992).
Author information
Authors and Affiliations
Corresponding author
Additional information
**Presented at the Second International Seminar on Molecular Spectroscopy and the Photochemistry of Macroheterocyclic Compounds, October 18–19, 2022, Minsk, Belarus.
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 90, No. 3, pp. 423–433, May–June, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stepuro, I.I., Ageiko, S.A., Stsiapura, V.I. et al. Nitration of Tyrosine and Tyrosyl Residues in Myoglobin by the Action of Visible Light in the Presence of Riboflavin and Nitrite**. J Appl Spectrosc 90, 543–553 (2023). https://doi.org/10.1007/s10812-023-01565-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-023-01565-z