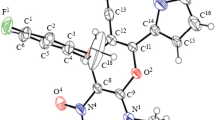
A microwave-assisted, chemoselective synthesis of novel antitumor and antimicrobial (3E)-5-hydroxy-1-isopropyl-3-[(5-methyl-2-thienyl)methylene]-5-phenylpyrrolidin-2-one has been achieved via the solvent-free one-pot reaction of (3E)-3-[(5-methyl-2-thienyl)methylene]-5-phenylfuran-2(3H)-one with isopropylamine. The product is obtained in significant purity and yield under ecofriendly reaction conditions. The microwave technique surpasses conventional thermal heating approaches by accelerating the reaction in a clean, ecofriendly manner that avoids the use of organic or toxic solvents. The structural formula of the product is confirmed by crystallographic and spectroscopic characterization. X-ray single crystal diffraction reveals that the compound crystallizes in an orthorhombic centrosymmetric crystal form, with unambiguous assignment of the E-configuration for the C3–Cthienyl bond. The synthesized molecules have two centers of chirality in the hydroxypyrrolidin-2-one ring: 1) the carbon atom attached to nitrogen, the hydroxyl group, and the phenyl ring; 2) the nitrogen atom attached to the carbonyl carbon R3 group, the chiral carbon in the ring, and the covalent bond bearing the lone pair of electrons. The molecular geometry is also optimized using density functional theory calculations, and the results obtained are in good agreement with the experimental data. Evaluation of the biological and medicinal activity of the compound affords similar results to the reference data in antitumor treatment of human colon and breast cells, which can be attributed to the presence of the hydroxyl group, the heterocyclic motifs, and sulfur. Calculation of the molecular electrostatic potential locates the most electrophilic site near the hydroxyl group attached to the heterocyclic ring, which is consistent with the bioactivity results. The frontier molecular orbitals are also determined, finding that the energy difference between highest occupied molecular orbital and lowest unoccupied molecular orbital is −0.15228 eV. A mechanism is proposed in which an intramolecular nucleophilic attack occurs on the carbonyl carbon by the lone pair of electrons on the nitrogen atom, leading to ring closure with proton transfer to oxygen and final formation of the hydroxyl group.
Similar content being viewed by others
References
V. O. Koz’minykh, N. M. Igidov, S. S. Zykova, V. É. Kolla, N. S. Shuklina, and T. F. Odegova, Pharm. Chem. J., 36, 188–191 (2002).
T. Michael, A. Michael, T. Andreas, H. Ulrich, B. Mirko, and N. A. Johannes, Patent WO2008055945(A1) (2008).
Y. Geng, X. Wang, L. Yang, H. Sun, Y. Wang, Y. Zhao, R. She, M.-X. Wang, D.-X. Wang, and J. Tang, PLoS One, 10, 1–15 (2015).
A. Pendri, T. L. Troyer, M. J. Sofia, M. A. Walker, B. N. Naidu, J. Banville, N. A. Meanwell, I. Dicker, Z. Lin, M. Krystal, and S. W. Gerritz, J. Comb. Chem., 12, 84–90 (2010).
K. Ma, P. Wang, W. Fu, X. Wan, L. Zhou, Y. Chu, and D. Ye, Bioorg. Med. Chem. Lett., 21, 6724–6727 (2011).
V. L. Gein, M. N. Armisheva, N. A. Rassudikhina, M. I. Vakhrin, and E. V. Voronina, Pharm. Chem. J., 45, 162–164 (2011).
V. L. Gein, V. A. Mihalev, N. N. Kasimova, E. V. Voronina, M. I. Vakhrin, and E. B. Babushkina, Pharm. Chem. J., 41, 208–210 (2007).
V. L. Gein, V. V. Yushkov, N. N. Kasimova, N. S. Shuklina, M. Y. Vasil’eva, and M. V. Gubanova, Pharm. Chem. J., 39, 484–487 (2005).
M. S. F. Franco, G. A. Casagrande, C. Raminelli, S. Moura, M. Rossatto, F. H. Quina, C. M. P. Pereira, A. F. C. Flores, and L. Pizzuti, Synth. Commun., 45, 692–701 (2015).
M. Anada and S. Hashimoto, Tetrahedron Lett., 39, 79–82 (1998).
D.-R. Choi, K.-Y. Lee, Y.-S. Chung, J.-E. Joo, Y.-H. Kim, C.-Y. Oh, Y.-S. Lee, and W.-H. Ham, Arch. Pharm. Res., 28, 151–158 (2005).
L. E. Burgess and A. I. Meyers, J. Org. Chem., 57, 1656–1662 (1992).
L. E. Overman and T. P. Remarchuk, J. Am. Chem. Soc., 124, 12–13 (2002).
V. Singh, R. Saxena, and S. Batra, J. Org. Chem.,70, 353–356 (2005).
R. Sarkar and C. Mukhopadhyay, Tetrahedron Lett., 54, 3706–3711 (2013).
J. Sun, Q. Wu, E.-Y. Xia, and C.-G. Yan, Eur. J. Org. Chem., 16, 2981–2986 (2011).
Q. Zhu, H. Jiang, J. Li, S. Liu, C. Xia, and M. Zhang, J. Comb. Chem., 11, 685–696 (2009).
B. M. Awad, H. A. Saad, E. M. Nassar, and E. M. Azmy, J. Am. Sci., 9, 566–577 (2013).
P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney, and M. R. Boyd, J. Natl. Cancer Inst., 82, 1107–1112 (1990).
H. D. Flack and G. Bernardinelli, Chirality, 20, 681–690 (2008).
N. R. Guirguis, B. M. Awad, and H. A. Saad, J. Pract. Chem., 332, 414–418 (1990).
N. R. Guirguis, B. M. Awad, and H. A. Saad, Liebigs Ann. Chem., No. 6, 1003–1011 (1986).
A. Lausi, M. Polentarutti, S. Onesti, J. R. Plaisier, E. Busetto, G. Bais, L. Barba, A. Cassetta, G. Campi, D. Lamba, A. Pifferi, S. C. Mande, D. D. Sarma, S. M. Sharma, and G. Paolucci, Eur. Phys. J. Plus., 130, 1–8 (2015).
W. Kabsch, Acta Crystallogr. Sect. D Biol. Crystallogr., 66, 125–132 (2010).
G. M. Sheldrick, Acta Crystallogr. Sect. A Found. Adv., 71, 3–8 (2015).
P. W. Betteridge, J. R. Carruthers, R. I. Cooper, K. Prout, and D. J. Watkin, J. Appl. Crystallogr., 36, 1487–1489 (2003).
A. L. Spek, Acta Crystallogr. Sect. D Biol. Crystallogr., 65, 148–155 (2009).
L. J. Farrugia, J. Appl. Crystallogr., 45, 849–854 (2012).
K. Brandenburg, Cryst. Impact GbR (2012).
I. J. Bruno, J. C. Cole, M. Kessler, J. Luo, W. D. S. Motherwell, L. H. Purkis, B. R. Smith, R. Taylor, R. I. Cooper, S. E. Harris, and A. G. Orpen, J. Chem. Inform. Comput. Sci., 44, 2133–2144 (2004).
R. Ditchfield, W. J. Hehre, and J. A. Pople, J. Chem. Phys., 54, 724–728 (1971).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
J. B. Foresman and A. Frisch, Exploring Chemistry with Electronic Structure Methods, Gaussian, Inc, Pittsburgh, P.A. (1996).
Gaussian09, Rev., Gaussian, Inc., Wallingford CT (2009).
A. Frisch, A. B. Nielson, and A. J. Holder, Gauss View User Manual, Gaussian Inc., Pittsburgh, Pennsylvania (2005).
B. D. Joshi, R. Mishra, P. Tandon, A. C. Oliveira, and A. P. Ayala, J. Mol. Struct., 1058, 31–40 (2014).
Munoz-Caro, Nino, Senent, Leal, and Ibeas, J. Org. Chem., 65, 405–410 (2000).
S. Radhakrishnan, R. Parthasarathi, V. Subramanian, and N. Somanathan, Comput. Mater. Sci., 37, 318–322 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Abstract of article is published in Zhurnal Prikladnoi Spektroskopii, Vol. 88, No. 2, p. 334, March–April, 2021.
Rights and permissions
About this article
Cite this article
Azmy, E.M., Awad, B.M., Hefni, H.A. et al. Efficient One-Pot Microwave-Assisted Synthesis, Crystallographic, and Spectroscopic Characterization of Novel Antitumor and Antimicrobial (3E)-5-Hydroxy-1-Isopropyl-3-[(5-Methyl-2-Thienyl)Methylene]-5-Phenylpyrrolidin-2-One. J Appl Spectrosc 88, 414–423 (2021). https://doi.org/10.1007/s10812-021-01189-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-021-01189-1