Abstract
Seaweed farming is expanding in Europe and may provide environmental benefits similar to those from natural kelp forests and shellfish farms, including habitat provisioning. Few studies have substantiated these claims however, and it remains uncertain whether seaweed farms will support similar biodiversity to kelp forests or provide valuable long-term habitat beyond the harvest season. We repeatedly surveyed an integrated sugar kelp (Saccharina latissima) and blue mussel (Mytilus edulis) farm in southwest UK to compare epibiont assemblages between cultivated kelps, to those from three nearby wild kelp populations, and to epibionts on farmed mussel lines and unseeded ‘bare’ lines. We found farmed kelps supported over 217 times the abundance of epibionts living on wild kelps at harvest time, however, taxonomic diversity per kelp was lower at the farm. Farmed kelp assemblages were dominated by amphipods, which were present on the wild kelps but in much lower numbers. Farmed kelp also supported distinct assemblages to cultivated mussels, which were similarly dominated by amphipods, but hosted higher relative abundances of crabs, echinoderms, worms and red algal biomass. The bare lines were heavily colonised by another pseudo-kelp, Saccorhiza polyschides, which supported similar epibiont assemblages to the seeded S. latissima lines. Our findings indicate that cultivating bivalves alongside seaweed can increase habitat provisioning at a seaweed farm and extend its permanence beyond typical seaweed cultivation periods as bivalves have longer, continuous farming periods. However, the presence of mussels will likely influence the epibiont assemblages on the farmed kelp, which are distinct from wild kelp populations.
Similar content being viewed by others
Introduction
Seaweed farming is increasing in Europe due to a rising demand for seaweed products, and growing recognition of its potential environmental benefits and contributions to ecosystem services (Wood et al. 2017; Hasselström et al. 2018; Alleway et al. 2019; Langton et al. 2019; Gentry et al. 2020). Environmental benefits from seaweed farming could include improving water quality and providing new habitat for invertebrate and fish species, similar to wild kelp populations and cultivated bivalve shellfish (Theuerkauf et al. 2021; Corrigan et al. 2022; Forbes et al. 2022). Habitat creation may be particularly important in coastal ecosystems where pre-existing natural habitats have been removed or degraded by human activities such as pollution, climate change, or certain destructive fishing practices (Barbier et al. 2011). ‘Restorative’ or ‘regenerative aquaculture’, whereby farms are sustainably managed to deliver ecosystem services, could provide a valuable refuge or starting point for coastal biodiversity to recover in degraded areas, while generating marketable products and livelihoods to boost local economies (Theuerkauf et al. 2019; Overton et al. 2023). Currently, however, few studies have investigated habitat provisioning at seaweed farms. It is also unclear whether farms offer valuable habitat compared to wild seaweed-dominated habitats (e.g., kelp forests), or whether farms will potentially alter wider ecosystem dynamics by harbouring different species and supporting distinct communities (Corrigan et al. 2022; Forbes et al. 2022).
Naturally-occurring kelp forests represent some of most productive habitats on Earth and act as important repositories of marine biodiversity that support diverse flora and fauna (Smale et al. 2013; Teagle et al. 2017; Bué et al. 2020; King et al. 2021; Salland and Smale 2021). Similarly, cultivated seaweed (especially large kelp species) could create valuable new habitats by providing complex three-dimensional living space, elevated food supply and enhanced reproduction and recruitment opportunities (Campbell et al. 2019; Theuerkauf et al. 2021; Corrigan et al. 2022). A range of epibiotic organisms can rapidly colonise available surfaces at farm sites, including aquaculture infrastructure or cultivated seaweed, and are generally considered pests by farmers as they have detrimental effects on crop quality and farming operations (Bannister et al. 2019). However, not all colonising epibionts are permanent or damaging, and some taxa, such as bryozoans, bivalves, sponges, tunicates, and other algae, may provide additional environmental benefits to farms. These benefits include improving water quality through biofiltration and nutrient regulation (Hurd et al. 1994; Pica et al. 2019; Montalto et al. 2020), or as food sources for higher trophic levels such as fish and macroinvertebrates (Theuerkauf et al. 2021; Corrigan et al. 2022). This in turn could potentially support secondary food production with spill-over benefits for fisheries (Gentry et al. 2020).
Recent studies in Europe have found that cultivated kelp provides distinct, novel habitat compared to wild kelp populations and supports dissimilar associated communities (Walls et al. 2016; Bekkby et al. 2023). In Ireland, higher levels of epibiont biodiversity were associated with cultivated Laminaria digitata holdfasts compared to those from neighbouring wild populations (Walls et al. 2016), whereas in Norway, cultivated Saccharina latissima and Alaria esculenta supported lower epibiont biodiversity, abundance and richness compared to wild kelps, despite hosting similar species (Bekkby et al. 2023). The differences in these findings, and other recent understandings of how epibiont assemblages vary widely between cultivation sites, dependent on latitude and environmental factors such as temperature, flow regime and wave exposure (Matsson et al 2019; Forbord et al. 2020; Visch et al. 2020a, b; Boderskov et al. 2021), highlight the need to quantify epibionts effectively at multiple sites, particularly in emerging areas for the industry, such as across Europe. Furthermore, most studies have focused on epibionts present at, or before harvesting time points (e.g. Bekkby et al. 2023), which is before epibiont assemblages have fully developed on annually-seeded farms (Corrigan et al. 2023). Typical kelp cultivation seasons in the UK and Europe (late autumn to beginning of summer, ~ 7 months) would indicate farms only provide temporary habitat compared to wild kelp populations, which can persist for decades (Forbes et al. 2022). For seaweed farming to provide valuable long-term habitat, innovations to maintain biodiversity beyond the harvest point are being trialled, including partial harvesting techniques (Corrigan et al. 2023). Co-location with other marine industries, such as renewable energy, or longer-lived aquaculture species, such as bivalve shellfish, can also increase the longevity of habitat.
Co-cultivation or integrated multi-trophic aquaculture (IMTA) of species that rely on similar infrastructure and management is increasing in popularity in Europe (Alexander et al. 2015; Hughes and Black 2016; Kleitou et al. 2018). Growing low-trophic species, such as seaweed and bivalves together, where neither culture requires additional food, fertilisers or fresh water to grow, uses space more efficiently in coastal waters and may also have mutual benefits to both cultures, such as biofiltration and improving water quality and clarity, enhancing primary production, and reducing the settlement of nuisance biofouling species (Holdt and Edwards 2014; Hargrave et al. 2021, 2022; Jiang et al. 2022). The proposed environmental benefits of seaweed farming are largely similar to those of bivalve shellfish production (Holdt and Edwards 2014; Hughes and Black 2016; Campbell et al. 2019), however, shellfish farms have received more study. For instance, in a recent review evaluating habitat provisioning by seaweed and bivalve farms, only eight out of 65 studies were conducted at seaweed farms (Theuerkauf et al. 2021). The review found that both bivalve and seaweed farms are associated with high abundance and diversity of mobile macrofauna, however, bivalve farms hosted higher abundances and species richness. Nevertheless, few, if any, direct comparisons have been made between cultivated species at individual farms. It also remains unclear whether epibiont species are more attracted to the farm infrastructure or the farmed biomass (Powers et al. 2007; Theuerkauf et al. 2021; Corrigan et al. 2022). A study in Ireland compared unseeded, or bare ropes, to those seeded with A. esculenta and found different epibiont assemblages between them, indicating that cultivated kelps offer distinct habitat and potentially suppress other algae from settling (Walls et al. 2019). Similarly in Norway, farmed kelp and bare ropes hosted distinct faunal assemblages (Bekkby et al. 2023), highlighting how farm infrastructure alone may influence habitat provisioning at aquaculture sites.
This study aims to compare epibiont assemblages across farmed kelp to wild populations, cultivated mussels and bare farm infrastructure to determine whether seaweed farms provide similar and valuable coastal habitat. We repeatedly surveyed an integrated sugar kelp (Saccharina latissima) and blue mussel (Mytilus edulis) farm in southwest UK, collecting cultivated biomass from seaweed, mussel and bare lines, and individual S. latissima plants from three nearby wild kelp populations. We compared farmed and wild S. latissima plants at normal harvest time (May–June) and beyond harvest (August) to assess whether epibiont assemblages would become more similar to wild populations with time. We expected the farmed S. latissima to host distinct assemblages to the wild S. latissima and the farmed mussel and bare lines, as they provide a new temporary suspended substrate and food source in the water column. Increased understanding of the epibiont communities associated with seaweed farming will help determine whether farms can act as “restorative aquaculture” sites that may have wider implications for secondary food production available to fish and other species. Given the degradation of coastal habitats and the cost of their restoration, understanding how aquaculture can be designed to have enhanced positive environmental benefits and further contribute to ecosystem services is essential in ensuring ecological, social and economic sustainability of our coastlines (Theuerkauf et al. 2019).
Methods
Study sites
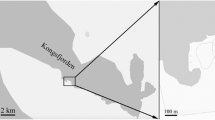
Sugar kelp (Saccharina latissima), blue mussel (Mytilus edulis), and unseeded ‘bare’ rope samples were collected from an integrated multi-trophic aquaculture (IMTA) site in Porthallow Bay, Cornwall, UK (50° 04' N, 5° 04' W) (Fig. 1). Porthallow Bay is exposed to wind and wave action from the east and southeast but is sheltered from the predominant south-westerly winds and North Atlantic swells, with the 16 ha integrated farm site situated at approximately 500 m from the shore (Corrigan et al. 2023). The farm uses a longline system with 200 m header lines anchored to the seabed and suspended 1–3 m below the surface, supporting either seaweed or mussel growth (Fig. 1). The farm is suspended over seabed depths of 8–15 m (below chart datum), with the seabed below consisting of mixed rocky substrate, soft sediments and maerl gravel.
Study site map with A) location of the study region in southwest UK; B) location of Porthallow Bay in Cornwall, UK; C) location of farm site and wild kelp collection sites 1, 2 & 3; D) diagram of the farm system suspending seaweed, mussel and bare lines. N.B. Seaweed and bare lines were set on separate headerlines to the mussels as shown in C, not interspersed as shown in D. Seaweed and mussel graphics are from Biorender (biorender.com)
Seaweed lines were seeded in late November 2019 and late October 2020, with S. latissima gametophytes attached directly onto ~ 6 m long dropper lines (braided 12 mm AlgaeRope, AtSeaNOVA, Belgium) using a binder solution (AtSeaNOVA). Seeded droppers were then spaced at 2–3 m apart along the header lines. Unseeded (hereafter ‘bare’) lines of the same material and length were also set out alongside the seeded seaweed lines in November 2019. Mussel dropper lines (10 m braided fibrous ropes with plastic pegs spaced at 1 m intervals to prevent slippage) were set ~ 1 m apart on the header lines suspended from April 2019. Mussel lines were seeded following the natural spawning and settlement cycle of M. edulis, whereby as seawater temperatures rise (typically from March to May), mussels spawn and their larvae then settles naturally onto the dropper ropes as spat and continue to grow for ~ 18 months before harvest, by which time new spat will have settled for the next harvesting season.
Wild S. latissima populations were sampled at three nearby shallow subtidal reef sites (~ 2–4 m depth), which experience similar environmental conditions to the cultivated kelps and are within 2.5 km of the farm site and each other (Fig. 1).
Sample collection
Individual kelp plants were collected in May (2021) and August (2020) from both the farm and nearby wild populations (Fig. 1). Collections in May represented ‘harvest’ assemblages and those in August were indicative of ‘post-harvest’ assemblages, if and when kelp biomass is not removed from the farm to assess whether epibiont assemblages would become more similar to wild populations with time (Corrigan et al. 2023). In May 2021, 18 kelp plants were collected (by boat) from three independent droppers spread along one seaweed header line. From each dropper, three representative plants were randomly selected from both 0–1 m and 3–4 m depth increments to capture any variability with depth. Once removed, plants were placed into separate labelled sealable bags. In August 2020, a total of 36 kelp plants were sampled in the same way (Corrigan et al. 2023). Ten wild kelps from natural populations, comparable in size to the farmed kelps, were collected from each rocky reef site in May 2021 and August 2020 using SCUBA. Before removing the holdfast from the reef, cotton bags were placed over the entire plant and then sealed. All samples were kept on ice and then frozen at -20 °C within eight hours of sampling and processed at a later date.
To compare epibiont assemblages between cultivated S. latissima to M. edulis cultivation lines and bare unseeded lines, in June 2020, 0.5 m sections of each line type were cut from 2–2.5 m depth, consistent with the growing depth of both species. Three replicate sections from each line type were collected. Samples were placed in sealed bags and frozen at -20 °C within 4 h of collection.
Sample processing
All kelp samples were processed as per Corrigan et al. (2023), whereby plants were defrosted and rinsed through a 0.5 mm sieve to remove any mobile or loosely attached fauna (hereafter referred to as ‘mobile’ epibionts). Samples were then thoroughly examined for any remaining sedentary or sessile individuals and colonial taxa (e.g. bryozoans, ascidians, algae) (hereafter referred to as ‘sessile’ epibionts) on both sides of blades and in the interstitial spaces of holdfasts, which were carefully removed where possible. All taxa were sorted into coarse taxonomic groups (Table S1), enumerated, and weighed (wet weight). Colony-forming taxa and mat-forming algae that could not be easily removed were quantified by estimating percent cover of the blade (Table S1). Taxa richness was defined as the number of taxa present in a sample (Table S1).
We quantified the morphological characteristics of sampled kelp plants (e.g., habitable holdfast volume, blade surface area), as species richness often scales with habitat size (Anderson et al. 2005). Saccharina latissima samples were measured to attain total plant biomass, maximum blade length and width, holdfast habitable volume, and biomass of blade and holdfast individually. Blade surface area was calculated approximately using maximum blade width x maximum blade length. Habitable volume within holdfasts was calculated using displacement (as described in Teagle et al. 2018), by first measuring the volume of water displaced by the holdfast, then subtracting this from the volume of water displaced by the holdfast wrapped in plastic food wrap.
The 0.5 m sections of S. latissima, M. edulis and bare lines were processed as above, whereby samples were defrosted, rinsed for mobile taxa and inspected for sessile and colonial taxa. Once epibionts had been removed, the biogenic habitat forming biomass (either mussel or kelp) was weighed. As expected, some S. latissima had settled on the M. edulis lines and vice versa, however, the bare lines had been almost completely colonised by other kelps, predominantly the pseudo-kelp Saccorhiza polyschides, which was also treated as a biogenic habitat-former as wild S. polyschides has been found to host significant epibiont biodiversity (Salland and Smale 2021).
Statistical analysis
The statistical approaches described below involve univariate and multivariate permutational analyses using the PERMANOVA add on for Primer v7 software (Anderson et al. 2008; Clarke and Gorley 2015).
For comparisons between farmed and wild kelps, differences in kelp morphology (holdfast habitable volume, blade surface area, and total kelp weight), and univariate assemblage metrics (total taxa richness, mobile epibiont abundance, mobile and algal epibiont biomass, and total sessile epibiont blade coverage per kelp) between sites and months were examined using two-way permutational analyses of variance (PERMANOVA) with “month” and “site” as fixed factors. For comparisons between line types at the farm, differences in habitat forming biomass and univariate assemblage metrics (total taxa richness, mobile epibiont abundance, and mobile and algal epibiont biomass) were examined using one-way PERMANOVA, including “line type” as a fixed factor. For each univariate comparison, PERMANOVAs with permutations (999 under an unrestricted model) were based on Euclidean distances between untransformed data. Pair-wise tests in PERMANOVA were then conducted wherever the main effect or interaction was significant (p < 0.05). For the line type comparisons, due to small sample sizes, Monte Carlo simulations were applied to generate the p-values.
Variability in multivariate assemblage structure between factors was examined using PERMANOVA and visualised using metric multidimensional scaling (mMDS) ordination. Multivariate assemblages were examined using the models described above, but with permutations based on separate Bray–Curtis resemblance matrices constructed from the following assemblage metrics: 1) the presence-absence of all taxa; 2) the abundance of mobile taxa; 3) the biomass of mobile taxa and easily detached algae; 4) the percent cover of sessile taxa (Corrigan et al. 2023). A presence-absence transformation was used for all taxa, as sessile species were not enumerated in the same way as mobile species (i.e. percent cover of colonies versus abundance of individuals). Fourth root transformation was chosen for abundance and biomass of mobile taxa to down-weight the influence of highly abundant amphipods. Square root transformation was used for percent coverage of sessile taxa to down-weight any highly abundant taxa. SIMPER analysis was then performed to determine which taxa contributed most to the observed dissimilarity for each assemblage metric. For both the univariate and multivariate metrics, differences in within-treatment variability between levels of factors were also examined using the permutational dispersion (PERMDISP) routine. Where within-treatment dispersion differed between groups, a more conservative p-value (p < 0.01) was adopted for the main PERMANOVA test for that given response variable (Anderson 2017).
Results
Comparisons between cultivated and wild S. latissima epibiont assemblages
Saccharina latissima plants were similar in biomass and holdfast habitable volume across the farm and wild sites in both May 2021 and August 2020 (i.e. were highly comparable for the purposed analyses, Fig. 2, Table 1). Blade surface areas at all sites were larger in May 2021 than August 2020, and wild site 3 had larger blades than the farm and wild site 1 in both months. Taxa richness per kelp was lower at the farm than all wild sites in both May and August. When considering total epibiont taxa richness across all sampled kelps however, the farm hosted similar taxa richness to the wild sites and the farm did not differ from wild site 1 in May (10 taxa), or wild site 2 in August (13 taxa). Epibiont abundance was over 16- and 217-fold higher at the farm than wild sites in August and May, respectively, due to the abundance of amphipods which dominated the farmed kelps (Fig. 3, Table 1), whereas much greater evenness was found at the wild sites in both months. Epibiont biomass was also higher on the farmed kelps compared to the wild sites in both May and August (due to the weight of the amphipods), but to a lesser degree than abundance, due to the presence of heavier Gastropoda and Rhodophyta epibionts on the kelps from the wild sites (Fig. 3, Table 1). Sessile epibiont blade coverage did not differ between the farm and wild sites in May, however in August, blade coverage at the farm was higher than all wild sites (Fig. 3, Table 1).
Mean kelp morphological biometrics of farmed and wild kelps (at three sites) in May 2021 and August 2020 for A) wet weight biomass; B) blade area; and C) holdfast habitable volume. Error bars are standard error (in May Farm, n = 18; August Farm, n = 36; in May and August, Wild 1,2,3, n = 10). Significant differences between sites within months are denoted with letters
Differences in epibiont assemblages of farmed and wild kelps (at three sites) in May 2021 and August 2020 in terms of A) taxa richness; B) percent cover of blade by sessile or mat forming epibionts; C) mobile or loosely attached epibiont abundance; D) mobile or loosely attached epibiont abundance percentage composition; E) mobile or loosely attached and algae epibiont biomass (excluding kelps); F) mobile or loosely attached and algae epibiont biomass percentage composition (excluding kelps). Bars are plotted with mean values per kelp (unless otherwise stated) and error bars represent standard error (in May Farm, n = 18; August Farm, n = 36; in May and August, Wild 1,2,3, n = 10). Significant differences between sites within months are denoted with letters
The multivariate analysis revealed differences in epibiont assemblages between the farmed and wild kelp plants for both survey months in terms of epibiont taxa presence-absence, abundance, biomass and sessile coverage of blades, although the farm did not differ in sessile coverage of blades between wild site 2 and 3 in May. Assemblages associated with wild kelp plants did vary between sites, however, there was often overlap as seen in the mMDS plots (Fig. 4) and Table 1. SIMPER analysis showed that differences between farmed and wild kelp-associated assemblages were often driven by amphipods and bryozoans, which were typically higher in the farm, while wild kelps supported higher proportions of gastropods, bivalves and worms (Fig. 3, Tables S2, S3, S4).
Metric MDS plots depicting multivariate analyses of epibiont assemblages of farmed and wild kelps in May 2021 and August 2020 in terms of A) presence-absence of total assemblage including mobile and sessile epibionts, B) percent coverage of blades by sessile taxa (square root transformed data with dummy variable = 0.7) C) abundance of mobile or loosely attached epibionts (fourth-root transformed), D) biomass for mobile or loosely attached epibionts and algae excluding kelps (fourth-root transformed). All plots are ordinated based on Bray–Curtis similarity matrices of taxa at coarse taxonomic level (i.e. phyla). In May Farm, n = 18; August Farm, n = 36; in May and August, Wild 1,2,3, n = 10
Comparisons between cultivated line types
Habitat forming biomass was greatest in the mussel lines, while the settlement of other kelp or pseudo-kelp species (predominantly S. polyschides) on the bare lines ensured there was no significant difference in habitat forming biomass between the bare lines and the lines seeded with S. latissima (which also had several S. polyschides plants growing among the S. latissima) (Fig. 5, Table 2). Epibiont taxa richness was highest on the mussel lines, with ~ 13 taxa per line and 16 taxa present in total, compared to 7–8 taxa per line and 9 taxa in total on both the seeded seaweed and bare lines respectively. It appeared that epibiont abundance and biomass were greatest on the bare lines, although this was found not to be statistically different, and all line types were dominated by amphipods (Fig. 5, Table 2).
Differences in line types for A) habitat forming biomass; B) epibiont taxa richness; C) presence-absence of total assemblages including mobile and sessile epibionts (metric MDS plot depicting multivariate analyses of epibiont assemblages); D) mobile or loosely attached epibiont abundance (excluding kelps); E) mobile or loosely attached and algae epibiont biomass (excluding kelps). Bars are plotted with mean values and error bars represent standard error (n = 3). MDS plot was ordinated based on Bray–Curtis similarity matrices of taxa at coarse taxonomic level (i.e. phyla). Significant differences between line types are denoted with letters
The multivariate analysis revealed differences in epibiont assemblages between the mussel lines and the seaweed and bare lines in terms of epibiont taxa presence-absence, abundance and biomass (Fig. 5, Table 2). SIMPER analysis showed that differences between the mussel lines and the seaweed and bare lines were driven by bivalves, echinoderms, decapods, worms and red algae, which were typically higher on the mussel lines (Fig. 5, Tables S6, S7, S8).
Discussion
This study presents the first comparison of epibiont assemblages between farmed and wild kelp and between farmed kelp and mussels in the UK, which like other European countries, has a growing cultivation industry for both seaweed and shellfish species (Capuzzo and McKie 2016; Hughes and Black 2016). We found that farmed S. latissima plants hosted similar taxa but also distinct assemblages to wild plants, and that this dissimilarity persisted even after farmed plants were left growing three months beyond the typical harvest point. This demonstrates that farmed kelps in the UK may provide distinct habitats compared to natural kelp populations. The farmed kelp supported much higher epibiont abundances, biomass and sessile coverage than wild kelps, predominantly from amphipods and bryozoans. This has potential for supporting secondary production at the farm site, by providing food for pelagic fish species and macroinvertebrates (Theuerkauf et al. 2021; Corrigan et al. 2022). The unseeded bare lines were heavily colonised by the opportunistic pseudo-kelp S. polyschides, which hosted similar epibiont assemblages (dominated by amphipods) to the farmed S. latissima lines. The mussel lines hosted higher taxa richness and higher relative abundances of crabs, echinoderms, worms and greater red algal biomass compared with the farmed seaweed and bare lines. Our findings indicate that cultivating bivalves alongside seaweed can increase habitat provisioning at a seaweed farm and extend its permanence beyond typical seaweed cultivation periods as bivalves have longer, continuous farming periods. However, the presence of mussels will likely influence the epibiont assemblages on the farmed kelp, which are distinct from wild kelp populations.
The epibiont taxa present in this study were consistent with those previously recorded on kelp plants at the farm in Porthallow Bay (Corrigan et al. 2023), as well as studies conducted elsewhere at comparable sites in Europe on both farmed (Peteiro and Freire 2013; Førde et al. 2016; Walls et al. 2016, 2017; 2019; Rolin et al. 2017; Bak et al. 2018; Forbord et al. 2020; Visch et al. 2020a, b; Bekkby et al. 2023) and wild plants (Arnold et al. 2016; Walls et al. 2016; Teagle et al. 2018; Bué et al. 2020; Salland and Smale 2021; Bekkby et al. 2023). This study also supports recent findings that kelp farms in Europe form distinct habitats to wild kelp forests, as there were consistent differences in epibiont assemblage structure and taxa richness between farmed and wild kelps (Walls et al. 2016; Bekkby et al. 2023). In Ireland, species richness was higher on cultivated rather than wild Laminaria digitata holdfasts (Walls et al. 2016), whereas in Porthallow Bay, farmed kelps supported lower taxa richness per kelp than wild kelps, despite overall taxa richness at the Porthallow farm being comparable to the wild sites in August. The difference in results between these studies may be due to the different kelp species or regions investigated, or because only assemblages associated with holdfasts were examined in Ireland, whereas assemblages associated with entire plants were examined here. In Norway entire plants of both S. latissima and A. esculenta exhibited lower faunal richness and lower overall biodiversity than wild kelp plants after both three and seven months of cultivation (Bekkby et al. 2023). Similarly, in Porthallow Bay epibiont assemblages on farmed kelp did not become more similar to those associated with wild kelp plants following an additional three-month growth period beyond their typical harvest time, suggesting that dissimilarity between assemblage types is persistent at the time frames studied.
Farmed kelp assemblages may be distinct from those hosted by wild kelps for several reasons. Farmed kelps are typically suspended in the top six metres of the water column, experience a constant water depth, and are attached to floating semi-mobile substrate, whereas wild kelp plants experience varying water depths (due to tides) and are often attached to fixed, stable substrate, resulting in a very different hydrodynamic environment (Walls et al. 2016; Bekkby et al. 2023). Suspended farmed kelps are also isolated, to some extent, from benthic habitats so that less dispersive species (e.g. those lacking a mero-planktonic stage or swimming capacity) are less likely to recruit to and colonise suspended cultivated plants (Walls et al. 2016). Natural kelp plants can persist for several years, allowing time for successional processes to occur and established communities to develop, whereas farmed kelps are typically removed after less than a year of growth (Christie et al. 1998; Walls et al. 2016; Bekkby et al. 2023). For instance, natural populations of S. latissima commonly occur as annuals, however some plants can survive for 2–4 years (White and Marshall 2007), whereas the farmed kelps in this study were sampled after seven (normal harvest time) and ten months of growth. In this study, where possible, we collected wild S. latissima plants of comparable size to those from the farm (indicated by the similarities in kelp biomass and habitable holdfast volume) however it is possible that wild kelp plants were older than farmed kelps as there is no simple method to accurately age S. latissima plants. Farmed kelps are also grown in much higher densities, typically consisting of only one or two species, with approximately 120 kelp plants per meter of line at the Porthallow farm (Corrigan et al. 2023), compared to those from mixed beds in natural kelp populations, where densities of mature plants are typically around ~ 10 per square meter (Smale et al. 2016; Smale and Moore 2017). In this study, wild S. latissima plants were observed to be relatively sparsely distributed and estimated at approximately one per square metre (personal observation). Differences in densities, hydrodynamic forces and attachment substrate may also lead to the development of different morphological characteristics in farmed kelps, which may explain the differences in blade surface area that were observed between sites in this study, with higher wave exposures previously being linked to smaller blade surface areas in S. latissima in Norway (Visch et al. 2020b). Differences in kelp morphologies may in turn lead to variability in epibiont assemblages (Walls et al. 2016). As such, differences in abiotic and biotic conditions are likely to drive dissimilarity in assemblage structure between farmed and wild kelp plants, with farmed kelps supporting higher epibiont abundances but lower taxa richness than wild kelps and explain why certain taxa such as amphipods can proliferate so successfully at a farm.
The proliferation of amphipods on farmed kelp in Porthallow Bay led to higher overall epibiont abundance and biomass values compared with wild populations, suggesting that the farm supports significant secondary production and may provide an elevated food supply for higher consumers, such as pelagic fish species (Theuerkauf et al. 2021; Corrigan et al. 2022). The observed proliferation of amphipods has not been recorded at other European kelp farms, which have supported either similar (Walls et al. 2016) or lower (Bekkby et al. 2023) levels of epifaunal abundance compared to neighbouring wild kelps. These previous studies recorded the presence of amphipods on both wild and farmed kelp, but in much lower abundances than the present study, with only 36 individuals recorded per farmed kelp in Norway (Bekkby et al. 2023), compared to over 6000 individuals per kelp in Porthallow Bay over a similar cultivation period. The amphipods recorded at the Porthallow farm were predominantly small suspension-feeding and tube-building peracarid crustaceans that can occur in dense aggregations as females brood their offspring, which then recruit to the immediate vicinity (Thiel and Vásquez 2000). Amphipods, such as Jassa falcata (a common species found at the Porthallow farm), generally reach maturity and fecundity earlier in warmer temperatures, with peak reproduction occurring between 10 and 14 °C (Nair and Anger 1979, 1980). Porthallow is at a lower latitude compared to most other European seaweed cultivation sites reported upon previously, so the proliferation of amphipods may be due to the warmer water temperatures experienced earlier in the season causing earlier settlement (Corrigan et al. 2023). Amphipods can tolerate a wide range of wave exposures and salinities (Hill 2000), but population density typically increases with greater wave exposure and turbidity (Moore 1972). Although Porthallow is sheltered from prevailing winds, it is an open bay and experiences a relatively high degree of wave exposure and tidal mixing, compared to other more sheltered seaweed farms (such as those in fjords and lochs) (Corrigan et al. 2023). This may also explain why there were higher amphipod abundances in the suspended and more exposed farm at Porthallow, rather than the neighbouring benthic and more sheltered wild kelp sites. Wave exposure and sea temperature can also influence bryozoan and colonial ascidian colonisation (Rolin et al. 2017; Visch et al. 2020a, b), which overtook hydrozoan coverage at the Porthallow farm later in the season (Corrigan et al. 2023) and were much higher on farmed kelp blades than in wild populations. As farmed kelps are also grown in much higher densities than wild kelps, this could make it easier for amphipods, bryozoans and ascidians to proliferate if the environmental conditions are suitable, particularly if predation rates from benthic consumers (e.g., gastropods on bryozoans) are much reduced in the suspended kelp plants.
Porthallow Bay differs from other farm sites in previous studies, as it is not exclusively a seaweed farm but rather an established mussel farm with some seaweed lines. It is therefore highly possible that the amphipods recorded on the farmed kelp originated from the mussel lines, which also supported high amphipod abundances that dominated their assemblages. Despite high amphipod abundances and other shared taxa between the line types, mussels supported higher taxa richness and distinct epibiont assemblages compared to both the cultivated seaweed and bare lines. This was predominantly driven by higher relative abundances of crabs, echinoderms and worms, and greater red algal biomass, on the mussel lines. These taxa are consistent with other bivalve farms in Europe (Callier et al. 2018; Mascorda Cabre et al. 2021), which may differ to those supported by farmed seaweeds, as bivalves are in situ for longer, allowing more time for succession and assemblage development. The bivalves themselves also offer a primary food source for carnivorous species such as gastropods, starfish, crabs and flatworms (Flimlin and Beall 1993), which can also explain the differences seen in functional groups of epibionts between seaweed and mussel lines. Generally, bivalve farms appear to host higher abundances and species richness than seaweed farms (Theuerkauf et al. 2021), although habitat provisioning has been quantified disproportionately less in seaweed farms, and few, if any, direct comparisons have been made between co-cultivated species within individual farms. Co-cultivating aquaculture species could increase overall diversity at farm sites (if distinct epibiont assemblages are seen between cultivars), and this would also help to maintain habitat provisioning of a site beyond the typical cultivation period for short-lived species, such as seaweeds. For instance, the cultivation period for kelps in Europe is typically 6–7 months, whereas as mussels are cultivated for approximately 1–2 years. In Europe, there is also growing interest in co-locating aquaculture sites to increase management efficiency and address coastal space and licencing issues, so understanding the habitat value of IMTA sites is important to enable a more ecosystem directed view of marine planning (Hughes and Black 2016; Kleitou et al. 2018).
The farm infrastructure itself could also help to maintain habitat provisioning at the site once S. latissima is harvested, as the bare infrastructure lines were heavily colonised by naturally-settling S. polyschides, which hosted similar epibiont assemblages (primarily amphipods) compared to the seeded S. latissima lines. Previously however, in Ireland, unseeded ropes had distinct epibiont assemblages to those seeded with A. esculenta (Walls et al. 2019), and similarly in Norway, bare ropes hosted distinct faunal assemblages with lower abundances and taxa richness than cultivated S. latissima and wild kelp forests (Bekkby et al. 2023). This indicates that cultivated kelps offer distinct habitat to farm infrastructure and potentially suppress other algae from settling on seeded lines (Walls et al. 2019). It is unclear why S. polyschides colonised the bare lines so readily in Porthallow compared to previous studies in Ireland and Norway, however it may have been due to the presence of natural S. polyschides donor populations nearby. S. polyschides has also been reported at a S. latissima farm in Spain, particularly when S. latissima seeding density was low (Peteiro et al. 2006). As an opportunistic, fast growing and pioneering species that can tolerate a range of environmental conditions, S. polyschides can colonise new habitats easily (Peteiro et al. 2006; Salland and Smale 2021). Wild S. polyschides individuals in the southwest UK have also been dominated by amphipods (Salland and Smale 2021), however in much lower abundances than those recorded in the farm. Future comparisons should be made between the suspended S. polyschides that settles on the farm with wild benthic S. polyschides in the area, to improve understanding of how the farm differs from UK kelp forests, which are often made up of multiple kelp species.
In order to determine the overall habitat value of an aquaculture site, benthic and mobile pelagic assemblages, such as macroinvertebrate and fish species, also need to be included (Corrigan et al. 2022). With the high abundance and biomass of epibionts present at Porthallow Bay, this could provide significant food for larger invertebrate and fish species, and therefore increase secondary production at the site (Theuerkauf et al. 2021). This should be studied in more detail, as supporting secondary production has important implications for the economic value of the habit provided by farm sites, as well as its ecological importance for restoring habitat for fish populations that might be depleted from overfishing in coastal areas. Changes in mobile fish and macroinvertebrates distributions and abundances will also influence local ecosystem dynamics.
In conclusion, seaweed farms can provide habitat that supports high abundances of epibiont species, which readily colonise cultivated kelps such as S. latissima. These epibionts in turn provide food sources for fish and macroinvertebrate species, although quantifying this requires further investigation. The epibiont assemblages present on farms, however, are distinct from those that occur in natural kelp populations. The habitat provided by most seaweed farms is also temporary, as cultivated biomass is typically entirely removed at harvest, so most farms will not deliver on promises of “restorative aquaculture” unless they are designed to maintain biodiversity beyond the harvesting period. Incentive schemes could encourage farmers to maintain habitat provisioning and increase environmental stewardship at their sites, through the provision of additional support and financial compensation, like the recent UK Sustainable Farming Incentive or the Farming Investment Fund for terrestrial farmers, which aims to reward sustainable farming practices that support food production and benefit the environment (Defra 2022a, b). Another possible method to maintain habitat provisioning beyond seaweed harvests would be to combine seaweed farms with other longer growing aquaculture species that persist year-round, such as bivalves, which also host higher epibiont taxa richness. Farm-associated assemblages would still likely differ to those associated with wild populations however, so caution should be taken as to not alter nearby communities and habitats or introduce invasive species. Given the degradation of coastal habitats and the cost of their restoration, understanding how industry activities that are increasing in coastal areas, such as aquaculture, can be designed to have positive environmental benefits and contribute to ecosystem services is essential to ensure ecological, social and economic sustainability of our coastlines (Theuerkauf et al. 2019).
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Alexander KA, Potts TP, Freeman S, Israel D, Johansen J, Kletou D, Meland M, Pecorino D, Rebours C, Shorten M, Angel DL (2015) The implications of aquaculture policy and regulation for the development of integrated multi-trophic aquaculture in Europe. Aquaculture 443:16–23
Alleway HK, Gillies CL, Bishop MJ, Gentry RR, Theuerkauf SJ, Jones R (2019) The ecosystem services of marine aquaculture: valuing benefits to people and nature. Bioscience 69:59–68
Anderson MJ (2017) Permutational multivariate analysis of variance (PERMANOVA). In: Balakrishnan N, Colton T, Everitt B, Piegorsch W, Ruggeri F, Teugels JL (eds) Wiley StatsRef: Statistics reference online, Wiley pp 1–15
Anderson MJ, Diebel CE, Blom WM, Landers TJ (2005) Consistency and variation in kelp holdfast assemblages: spatial patterns of biodiversity for the major phyla at different taxonomic resolutions. J Exp Mar Biol Ecol 320:35–56
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. University of Auckland and PRIMER-E, Plymouth
Arnold M, Teagle H, Brown MP, Smale DA (2016) The structure of biogenic habitat and epibiotic assemblages associated with the global invasive kelp Undaria Pinnatifida in comparison to native macroalgae. Biol Invasions 18:661–676
Bak UG, Mols-Mortensen A, Gregersen O (2018) Production method and cost of commercial-scale offshore cultivation of kelp in the Faroe Islands using multiple partial harvesting. Algal Res 33:36–47
Bannister J, Sievers M, Bush F, Bloecher N (2019) Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 35:631–648
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193
Bekkby T, Torstensen RRG, Grünfeld LAH, Gundersen H, Fredriksen S, Rinde E, Christie H, Walday M, Andersen GS, Brkljacic MS, Neves L, Hancke K (2023) “Hanging Gardens”—Comparing fauna communities in kelp farms and wild kelp forests. Front Mar Sci 10:233
Boderskov T, Nielsen MM, Rasmussen MB, Balsby TJS, Macleod A, Holdt SL, Sloth JJ, Bruhn A (2021) Effects of seeding method, timing and site selection on the production and quality of sugar kelp, Saccharina latissima: A Danish Case Study. Algal Res 53:102160
Bué M, Smale DA, Natanni G, Marshall H, Moore PJ (2020) Multiple-scale interactions structure macroinvertebrate assemblages associated with kelp understory algae. Divers Distrib 26:1–15
Callier MD, Byron CJ, Bengtson DA, Cranford PJ, Cross SF, Focken U, Jansen HM, Kamermans P, Kiessling A, Landry T, O’Beirn F, Petersson E, Rheault RB, Strand Ø, Sundell K, Svasand T, Wikfors GH, McKindsey CW (2018) Attraction and repulsion of mobile wild organisms to finfish and shellfish aquaculture: a review. Rev Aquac 10:924–949
Campbell I, Macleod A, Sahlmann C, Neves L, Funderud J, Øverland M, Hughes A, Stanley M (2019) The environmental risks associated with the development of seaweed farming in Europe - Prioritizing key knowledge gaps. Front Mar Sci 6:107
Capuzzo E, McKie T (2016) Seaweed in the UK and Abroad – Status, Products, Limitations, Gaps and CEFAS Role. Defra. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/546679/FC002I__Cefas_Seaweed_industry_report_2016_Capuzzo_and_McKie.pdf; accessed 14 April 2023
Christie H, Fredriksen S, Rinde E (1998) Regrowth of kelp and colonization of epiphyte and fauna community after kelp trawling at the coast of Norway. In: Baden S, Phil L, Rosenberg R, Strömberg JO, Svane I, Tiselius P (eds) Recruitment, Colonization and Physical-Chemical Forcing in Marine Biological Systems. Springer, Cham pp 49–58
Clarke KR, Gorley RN (2015) Getting Started with PRIMER V7. PRIMER-E, Plymouth
Corrigan S, Brown AR, Ashton IGC, Smale DA, Tyler CR (2022) Quantifying habitat provisioning at macroalgal cultivation sites. Rev Aquac 14:1671–1694
Corrigan S, Brown AR, Tyler CR, Wilding C, Daniels C, Ashton IGC, Smale DA (2023) Development and diversity of epibiont assemblages on cultivated sugar kelp (Saccharina latissima) in relation to farming schedules and harvesting techniques. Life 13:209
Defra (2022a) Funding for Farmers and Land Managers. Department for Environment, Food & Rural Affairs and Rural Payments Agency. https://www.gov.uk/guidance/funding-for-farmers; accessed 14 May 2023
Defra (2022b) Sustainable Farming Incentive Opens for Applications. Department for Environment, Food & Rural Affairs, Rural Payments Agency, and The Rt Hon Victoria Prentis KC MP. https://www.gov.uk/government/news/sustainable-farming-incentive-opens-for-applications; accessed 14 May 2023
Flimlin G, Beall BF (1993) Major predators of cultured shellfish. Northeast Regional Aquaculture Center (NRAC) Bulletin No. 180
Forbes H, Shelamoff V, Visch W, Layton C (2022) Farms and forests: Evaluating the biodiversity benefits of kelp aquaculture. J Appl Phycol 34:3059–3067
Forbord S, Matsson S, Brodahl GE, Bluhm BA, Broch OJ, Handå A, Metaxas A, Skjermo J, Steinhovden KB, Olsen Y (2020) Latitudinal, seasonal and depth-dependent variation in growth, chemical composition and biofouling of cultivated Saccharina latissima (Phaeophyceae) along the Norwegian Coast. J Appl Phycol 32:2215–2232
Førde H, Forbord S, Handå A, Fossberg J, Arff J, Johnsen G, Reitan KI (2016) Development of bryozoan fouling on cultivated kelp (Saccharina Latissima) in Norway. J Appl Phycol 28:1225–1234
Gentry RR, Alleway HK, Bishop MJ, Gillies CL, Waters T, Jones R (2020) Exploring the potential for marine aquaculture to contribute to ecosystem services. Rev Aquac 12:499–512
Hargrave MS, Ekelund A, Nylund GM, Pavia H (2021) filtration and fertilisation effects of the bivalves Mytilus edulis and Magallana gigas on the kelp Saccharina Latissima in tank culture. J Appl Phycol 33:3927–3938
Hargrave MS, Nylund GM, Enge S, Pavia H (2022) Co-cultivation with blue mussels increases yield and biomass quality of kelp. Aquaculture 550:737832
Hasselström L, Visch W, Gröndahl F, Nylund GM, Pavia H (2018) The impact of seaweed cultivation on ecosystem services - a case study from the west coast of Sweden. Mar Pollut Bull 133:53–64
Hill J (2000) Jassa falcata an amphipod. In: Tyler-Walters H, Hiscock K (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews. Marine Biological Association of the United Kingdom, Plymouth
Holdt SL, Edwards MD (2014) Cost-effective IMTA: A comparison of the production efficiencies of mussels and seaweed. J Appl Phycol 26:933–945
Hughes AD, Black KD (2016) Going beyond the search for solutions: Understanding trade-offs in european integrated multi-trophic aquaculture development. Aquac Environ Interact 8:191–199
Hurd CL, Durante KM, Chia FS, Harrison PJ (1994) Effect of bryozoan colonization on inorganic nitrogen acquisition by the kelps Agarum fimbriatum and Macrocystis integrifolia. Mar Biol 121:167–173
Jiang L, Jansen HM, Broch OJ, Timmermans KR, Soetaert K (2022) Modelling spatial variability of cultivated Saccharina latissima in a Dutch coastal bay shows benefits of co-cultivation with shellfish. ICES J Mar Sci 79:2324–2335
King NG, Moore PJ, Wilding C, Jenkins HL, Smale DA (2021) Multiscale spatial variability in epibiont assemblage structure associated with stipes of kelp Laminaria hyperborea in the Northeast Atlantic. Mar Ecol Prog Ser 672:33–44
Kleitou P, Kletou D, David J (2018) Is Europe ready for integrated multi-trophic aquaculture? A survey on the perspectives of European Farmers and scientists with IMTA experience. Aquaculture 490:136–148
Langton R, Augyte S, Price N, Forster J, Noji T, Grebe G, St. Gelais A, Byron CJ (2019) An ecosystem approach to the culture of seaweed. NOAA Technical Memorandum NMFS-F/SPO-195
Mascorda Cabre L, Hosegood P, Attrill MJ, Bridger D, Sheehan EV (2021) Offshore longline mussel farms: A review of oceanographic and ecological interactions to inform future research needs, policy and management. Rev Aquac 13:1864–1887
Matsson S, Christie H, Fieler R (2019) Variation in biomass and biofouling of kelp, Saccharina latissima, cultivated in the Arctic, Norway. Aquaculture 506:445–452
Montalto V, Rinaldi A, Ape F, Mangano MC, Gristina M, Sarà G, Mirto S (2020) Functional role of biofouling linked to aquaculture facilities in Mediterranean enclosed locations. Aquac Environ Interact 12:11–22
Moore PG (1972) Particulate matter in the sublittoral zone of an exposed coast and its ecological significance with special reference to the fauna inhabiting kelp holdfasts. J Exp Mar Biol Ecol 10:59–80
Nair KKC, Anger K (1980) Seasonal variation in population structure and biochemical composition of Jassa falcata (Crustacea, Amphipoda) off the Island of Helgoland (North Sea). Estuar Coast Mar Sci 11:505–513
Nair KKC, Anger K (1979) Experimental studies on the life cycle of Jassa falcata (Crustacea, Amphipoda). Helgoländer Meeresun 32:444–452
Overton K, Dempster T, Swearer SE, Morris RL, Barrett LT (2023) Achieving conservation and restoration outcomes through ecologically beneficial aquaculture. Conserv Biol e14065
Peteiro C, Freire Ó (2013) Epiphytism on blades of the edible kelps Undaria pinnatifida and Saccharina latissima farmed under different abiotic conditions. J World Aquaculture Soc 44:706–715
Peteiro C, Salinas JM, Friere Ó, Fuertes C (2006) Cultivation of the autoctonus seaweed “Laminaria Saccharina” off the Galician Coast (NW Spain): Production and features of the sporophytes for an annual and biennial harvest. Thalassas 22:45–52
Pica D, Bloecher N, Dell’Anno A, Bellucci A, Pinto T, Pola L, Puce S (2019) Dynamics of a biofouling community in finfish aquaculture: A case study from the South Adriatic Sea. Biofouling 35:696–709
Powers MJ, Peterson CH, Summerson HC, Powers SP (2007) Macroalgal growth on bivalve aquaculture netting enhances nursery habitat for mobile invertebrates and juvenile fishes. Mar Ecol Prog Ser 339:109–122
Rolin C, Inkster R, Laing J, McEvoy L (2017) Regrowth and biofouling in two species of cultivated kelp in the Shetland Islands, UK. J Appl Phycol 29:2351–2361
Salland N, Smale DA (2021) Spatial variation in the structure of overwintering, remnant Saccorhiza polyschides sporophytes and their associated assemblages. J Mar Biol Assoc UK 101:639–648
Smale DA, Burrows MT, Evans AJ, King N, Sayer MDJ, Yunnie ALE, Moore PJ (2016) Linking environmental variables with regional-scale variability in ecological structure and standing stock of carbon within UK kelp forests. Mar Ecol Prog Ser 542:79–95
Smale DA, Burrows MT, Moore PJ, O’Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests : A Northeast Atlantic perspective. Ecol Evol 2090:4016–4038
Smale DA, Moore PJ (2017) Variability in kelp forest structure along a latitudinal gradient in ocean temperature. J Exp Mar Biol Ecol 486:255–264
Teagle H, Hawkins SJ, Moore PJ, Smale DA (2017) The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J Exp Mar Biol Ecol 492:81–98
Teagle H, Moore PJ, Jenkins H, Smale DA (2018) Spatial variability in the diversity and structure of faunal assemblages associated with kelp holdfasts (Laminaria hyperborea) in the Northeast Atlantic. PLoS One 13:e0200411
Theuerkauf SJ, Barrett LT, Alleway HK, Costa-Pierce BA, St. Gelais A, Jones RC (2021) Habitat value of bivalve shellfish and seaweed aquaculture for fish and invertebrates: Pathways, synthesis and next steps. Rev Aquac 14:54-72
Theuerkauf SJ, Morris JA, Waters TJ, Wickliffe LC, Alleway HK, Jones RC (2019) A global spatial analysis reveals where marine aquaculture can benefit nature and people. PLoS One 14:e0222282
Thiel M, Vásquez JA (2000) Are kelp holdfasts islands on the ocean floor? — Indication for temporarily closed aggregations of peracarid crustaceans. Hydrobiologia 440:45–54
Visch W, Kononets M, Hall POJ, Nylund GM, Pavia H (2020a) Environmental impact of kelp (Saccharina latissima) aquaculture. Mar Pollut Bull 155:110962
Visch W, Nylund GM, Pavia H (2020b) Growth and biofouling in kelp aquaculture (Saccharina latissima): The effect of location and wave exposure. J Appl Phycol 2:3199–3209
Walls AM, Edwards MD, Firth LB, Johnson MP (2019) Ecological priming of artificial aquaculture structures: Kelp farms as an example. J Mar Biol Assoc UK 99:729–740
Walls AM, Edwards MD, Firth LB, Johnson MP (2017) Successional changes of epibiont fouling communities of the cultivated kelp Alaria esculenta: Predictability and influences. Aquac Environ Interact 9:57–71
Walls AM, Kennedy R, Fitzgerald RD, Blight AJ, Johnson MP, Edwards MD (2016) Potential novel habitat created by holdfasts from cultivated Laminaria digitata: Assessing the macroinvertebrate assemblages. Aquac Environ Interact 8:157–169
White N, Marshall C (2007) Saccharina latissima sugar kelp. Marine life information network: biology and sensitivity key information reviews. https://www.marlin.ac.uk/species/detail/1375. Accessed 14 May 2023
Wood D, Capuzzo E, Kirby D, Mooney-McAuley K, Kerrison P (2017) UK macroalgae aquaculture: what are the key environmental and licensing considerations? Mar Policy 83:29–39
Acknowledgements
We like to thank the Cornish Seaweed Company and Westcountry Mussels of Fowey for their collaboration and access to the farm site. We would also like to thank Lily Burnet and Emma Stuart at the University of Plymouth and the Marine Biological Association for their help in sample processing.
Funding
This research was funded by the Worshipful Company of Fishmongers, Centre for Environment, Fisheries and Aquaculture Science (Cefas); the Marine Biological Association, European Maritime and Fisheries Fund, Research Council UK (RCUK) (through the Aquaculture Research Collaborative Hub—UK), South West Partnership for Environmental and Economic Prosperity (SWEEP) (NE/P011217/1), and the University of Exeter. D. A. Smale was supported by a UK Research and Innovation (UKRI) Future Leaders Fellowship (MR/S032827/1). The APC was funded by UKRI. For the purpose of open access, the author has applied a Creative Commons attribution (CC BY) licence to any author accepted manuscript version arising.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology, S. Corrigan, D. A. Smale, C. R. Tyler, R. A. Brown and I. G. C. Ashton; fieldwork, S. Corrigan, C. Wilding, C. Daniels, D. A. Smale; laboratory processing, S. Corrigan; data curation, S. Corrigan; formal analysis, S. Corrigan, D. A. Smale; writing- original draft preparation, S. Corrigan, D. A. Smale; writing- review and editing, C. R. Tyler, R. A. Brown, C. Wilding, C. Daniels, I. G. C. Ashton; visualization, S. Corrigan, D. A. Smale; supervision, D. A. Smale, C. R. Tyler, R. A. Brown and I. G. C. Ashton; project administration, S. Corrigan; funding acquisition, D. A. Smale, C. R. Tyler, R. A. Brown and I. G. C. Ashton, C. Daniels, C. Wilding. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Declarations
All animal work was carried out humanely and in accordance with the University of Exeter Ethical Approvals Committee.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corrigan, S., Brown, A.R., Tyler, C.R. et al. Home sweet home: Comparison of epibiont assemblages associated with cultivated and wild sugar kelp (Saccharina latissima), co-cultivated blue mussels (Mytilus edulis) and farm infrastructure. J Appl Phycol (2023). https://doi.org/10.1007/s10811-023-03055-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-023-03055-3