Abstract
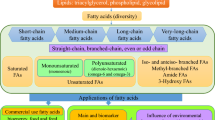
The integration of taxonomic and ecological traits is a more balanced approach in the systematic identification of living organisms. We isolated ten strains of Dunaliella from different geographical regions of Iran, including six terrestrial and four aquatic taxa. All strains belonged to the section Dunaliella based on the morphological and molecular investigation. First, optimal salinity was determined for bio-pigment accumulation. Each alga had its own optimum salinity, and the range of 1.5 to 2.5 M NaCl was further studied. Dunaliella parva YzS produced the maximum biomass density (231.92 mg L−1), while Dunaliella salina MrL accumulated the highest value of total carotenoid (1.72%AFDW). At the early stationary growth phase, the algae had a 11.7 to 41.7% lipid content. FAMEs varied from C10 to C24 with one or more double bonds. The results showed highly significant levels of palmitic (C16) and α-linolenic (C18:3n3) acids. According to the fatty acid profiles, the clustering pattern aligns with the sample origin. Compared to aquatic strains, terrestrials were more resistant to harsh conditions, e.g., dry conditions due to the synthesis of very long chain fatty acids. Lipid compositions may therefore be attributable to the environmental origins of taxa. The fatty acid composition was affected by the environmental conditions, while the accumulation of carotenoid was dependent on the biological and genetic entity of algal taxa. Examination of habitat parameters revealed that aquatic strains were well adapted to maintain homeostasis in higher salinity and conductivity; terrestrial strains were much better able to tolerate high concentrations of nitrate and phosphate.




Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed RA, He M, Aftab RA, Zheng S, Nagi M, Bakri R, Wang C (2017) Bioenergy application of Dunaliella salina SA 134 grown at various salinity levels for lipid production. Sci Rep-UK 7:1–10
Albertano P, Pinto G, Santisi S, Taddei R (1981) Spermatozopsis acidophila Kalina (Chlorophyta, Volvocales), a little known alga from highly acidic environments. Plant Biosyst 115:65–76
Amini M, Khoei ZA, Erfanifar E (2019) Nitrate (NO3−) and phosphate (PO43−) removal from aqueous solutions by microalgae Dunaliella salina. Biocatal Ag Biotechnol 19:101097
Arroussi HE, Benhima R, Elbaouchi A, Sijilmassi B, Mernissi NE, Aafsar A, Meftah-Kadmiri I, Bendaou N, Smouni A (2018) Dunaliella salina exopolysaccharides: a promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J Appl Phycol 30:2929–2941
Azúa-Bustos A, González-Silva C, Salas L, Palma RE, Vicuña R (2010) A novel subaerial Dunaliella species growing on cave spiderwebs in the Atacama Desert. Extremophiles 14:443–452
Ben-Amotz A, Avron M (1973) The role of glycerol in the osmotic regulation in the halophilic alga, Dunaliella parva. Plant Physiol 51:875–878
Ben-Amotz A, Avron M (1983) On the factors which determine the massive ß-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol 72:593–597
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917
Borowitzka LJ, Brown AD (1974) The salt relations of marine and halophilic species of the unicellular green alga Dunaliella. The role of glycerol as a compatible solute. Arch Microbiol 96:37–52
Borowitzka LJ, Kessly DS, Brown AD (1977) The salt relations in Dunaliella: further observations on glycerol production and its regulation. Arch Microbiol 113:131–138
Borowitzka MA (2013) Dunaliella: Biology, production, and markets. In: Richmond A, Hu Q (eds) Handbook of Microalgal Culture. John Wiley & Sons, London, pp 359–368
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825
Borowitzka MA, Borowitzka LJ (1988) Dunaliella. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal Biotechnology. Cambridge University Press, Cambridge, pp 27–58
Borowitzka MA, Borowitzka LJ, Kessly D (1990) Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J Appl Phycol 2:111–119
Borowitzka MA, Huisman JM (1993) The ecology of Dunaliella salina (Chlorophyceae, Volvocales): effect of environmental conditions on aplanospore formation. Bot Mar 36:233–244
Borowitzka MA, Siva CJ (2007) The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J Appl Phycol 19:567–590
Breckle SW (2002) Salt deserts in Iran and Afghanistan. In: Barth HJ, Böer B (eds) Sabkha Ecosystems Vol 1:the Arabian Peninsula and adjacent countries. Kluwer, Dordrecht, pp 109–122
Buchheim MA, Kirkwood AE, Buchheim JA, Verghese B, Henley WJ (2010) Hypersaline soil supports a diverse community of Dunaliella (Chlorophyceae). J Phycol 46:1038–1047
De Bigault Du, Granrut A, Cacas JL (2016) How very-long-chain fatty acids could signal stressful conditions in plants? Front Plant Sci 7:1490
Dhaka P, Singh GP (2016) Effect of salinity on growth and biopigment composition of Dunaliella salina isolated from Sambhar salt lake, Rajasthan, (India). Int J Pharm Bio Sci 7:528–533
Droop MR (1954) A note on the isolation of small marine algae and flagellates for pure cultures. J Mar Biol Assoc UK 33:511–514
Fang L, Qi S, Xu Z, Wang W, He J, Chen X, Liu J (2017) De novo transcriptomic profiling of Dunaliella salina reveals concordant flows of glycerol metabolic pathways upon reciprocal salinity changes. Algal Res 23:135–149
Fazeli MR, Tofighi H, Samadi N, Jamalifar H, Fazeli A (2006) Carotenoids accumulation by Dunaliella tertiolecta (Lake Urmia isolate) and Dunaliella salina (CCAP 19/18 & wt) under stress conditions. Daru 14:146–150
Gharajeh NH, Valizadeh M, Dorani E, Hejazi MA (2020) Biochemical profiling of three indigenous Dunaliella isolates with main focus on fatty acid composition towards potential biotechnological application. Biotechnol Rep 26:e00479
Gimmler H, Weis U (1992) Dunaliella acidophila - life at pH 1.0. In: Avron M, Ben-Amotz A (eds) Dunaliella: Physiology, Biochemistry, and Biotechnology. CRC Press, Boca Raton, pp 99–133
Ginzburg BZ, Ginzburg M (1985) Studies of the comparative physiology of the genus Dunaliella (Chlorophyta, Volvocales) 1. Response of growth to NaCl concentration. Brit Phycol J 20(3):277–283.
Gogna M, Choudhary A, Mishra G, Kapoor R, Bhatla SC (2020) Changes in lipid composition in response to salt stress and its possible interaction with intracellular Na+-K+ ratio in sunflower (Helianthus annuus L.). Environ Exp Bot, 178:104147.
Gomez PI, Gonzalez MA (2001) Genetic polymorphism in eight Chilean strains of the carotenogenic microalga Dunaliella salina Teodoresco (Chlorophyta). Biol Res 34:23–30
Goyal A (2007) Osmoregulation in Dunaliella, Part II: photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol Biochem 45:705–710
Graeve M, Kattner G, Wiencke C, Karsten U (2002) Fatty acid composition of Arctic and Antarctic macroalgae: indicator of phylogenetic and trophic relationships. Mar Ecol Prog Ser 231:67–74
Hejazi MA, Barzegari A, Gharajeh NH, Hejazi MS (2010) Introduction of a novel 18S rDNA gene arrangement along with distinct ITS region in the saline water microalga Dunaliella. Saline Systems 6:1–11
Jahnke LS (1999) Massive carotenoid accumulation in Dunaliella bardawil induced by ultraviolet-A radiation. J Photochem Photobiol B 48:68–74
Jahnke LS, White AL (2003) Long-term hyposaline and hypersaline stresses produce distinct antioxidant responses in the marine alga Dunaliella tertiolecta. J Plant Physiol 160:1193–1202
Jeffrey ST, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Juo ASR (1978) Selected methods for soil and plant analysis. IITA Mannual Series (Nigeria), no. 1 Ibadan, Oyo State (Nigeria). IITA. 52 p (No. 88–075105. CIMMYT.).
Kalina T (1965) Zur Morphologie und Taxonomie der Gattung Spermatozopsis Korschikow (Volvocales) Spermatozopsis acidophila sp. nov. Preslia 37:9–12
Kates M, Volcani BE (1966) Lipid components of diatoms. Biochim Biophys Acta-Lipid Lipid Met 116:264–278
Keerthi S, Koduru UD, Venkata SRD, Sarma NS (2016) Cysted forms of halophilic microalga Dunaliella salina under different stress conditions. Curr Sci India 111:261–262
Khalil ZI, Asker MM, El-Sayed S, Kobbia IA (2010) Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J Microbiol Biotech 26:1225–1231
Klug L, Daum G (2014) Yeast lipid metabolism at a glance. FEMS Yeast Res 14:369–388
Leonardi PI, Cáceres EJ (1997) Light and electron microscope observations of the life cycle of Dunaliella salina (Polyblepharidaceae, Chlorophyceae). Nova Hedwigia 64:621–633
Margulis L, Barghoorn ES, Ashendorf D, Banerjee S, Chase D, Francis S, Giovanonni S, Stolz J (1980) The microbial community in layered sediments at Laguna Figueroa, Baja California, Mexico: Does it have Precambrian analogues? Precamb Res 11:93–123
Matsumoto GI, Shioya M, Nagashima H (1984) Occurrence of 2-hydroxy acids in microalgae. Phytochemistry 23:1421–1423
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 265–284
Oren A (2005) A hundred years of Dunaliella research: 1905–2005. Saline Systems 1:1–14
Oren A (2014) The ecology of Dunaliella in high-salt environments. J Biol Res-Thessaloniki 21:1–8
Oren A, Shilo M (1985) Factors determining the development of algal and bacterial blooms in the Dead Sea: a study of simulation experiments in outdoor ponds. FEMS Microbiol Ecol 31:229–237
Pasiuga OS, Antonenko SP, Komaristaya VP, Rudas AN (2013) Variability of cultural and morphological traits of Dunaliella salina Teod. from different habitats. Journal of V.V. Karazin Kharkiv National University: Biology 18:54–63
Polat S, Ozogul Y (2008) Biochemical composition of some red and brown macro algae from the Northeastern Mediterranean Sea. Int J Food Sci Nutr 59:566–572
Prada F, Ayala-Diaz IM, Delgado W, Ruiz-Romero R, Romero HM (2011) Effect of fruit ripening on content and chemical composition of oil from three oil palm cultivars (Elaeis guineensis Jacq.) grown in Colombia. J Agr Food Chem 59:10136–10142
Raja R, Hemaiswarya S, Rengasamy R (2007) Exploitation of Dunaliella for β-carotene production. Appl Microbiol Biot 74:517–523
Roy SS, Pal R (2015) Microalgae in aquaculture: a review with special references to nutritional value and fish dietetics. Proc Zool Soc 68:1–8
Safarpour A, Amoozegar MA, Ventosa A (2018) Hypersaline environments of Iran: prokaryotic biodiversity and their potentials in microbial biotechnology. In: Egamberdieva D, Birkeland N-K, Panosyan H, Li W-J (eds) Extremophiles in Eurasian ecosystems: ecology, diversity, and applications. Springer, Singapore, pp 265–298
Sathasivam R, Kermanee P, Roytrakul S, Juntawong N (2012) Isolation and molecular identification of β-carotene producing strains of Dunaliella salina and Dunaliella bardawil from salt soil samples by using species-specific primers and internal transcribed spacer (ITS) primers. Afr J Biotechnol 11:16677–16687
Sathasivam R, Pongpadung P, Praiboon J, Chirapart A, Trakulnaleamsai S, Roytrakul S, Juntawong N (2018) Optimizing NaCl and KNO3 concentrations for high β-carotene production in photobioreactor by Dunaliella salina KU11 isolated from saline soil sample. Chiang Mai J Sci 45:106–115
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Srinivasan R, Mageswari A, Subramanian P, Suganthi C, Chaitanyakumar A, Aswini V, Gothandam KM (2018) Bicarbonate supplementation enhances growth and biochemical composition of Dunaliella salina V-101 by reducing oxidative stress induced during macronutrient deficit conditions. Sci Rep 8:1–14
Swofford D L (2002) PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods), Version 4.0b10. Sinauer Associates, Sunderland.
Tempesta S, Paoletti M, Pasqualetti M (2011) Morphological and molecular identification of a strain of the unicellular green alga Dunaliella sp. isolated from Tarquinia Salterns. Transitional Waters Bulletin 4(2):60–70.
Tran D, Mai T, Vo T, Ward A, Nguyen H, Hoang X (2014) Lipid signal can be an additional marker for the detection of Dunaliella salina. Wulfenia 21:216–233
Vaghefi SA, Keykhai M, Jahanbakhshi F, Sheikholeslami J, Ahmadi A, Yang H, Abbaspour KC (2019) The future of extreme climate in Iran. Sci Rep 9:1–11
Van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, Van der Werf MJ (2006) Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genom 7:142
Van den Hoek C, Mann D, Jahns HM, Jahns M (1995) Algae: an introduction to phycology. Cambridge University Press, Cambridge
Vanitha A, Narayan MS, Murthy KNC, Ravishankar GA (2007) Comparative study of lipid composition of two halotolerant alga, Dunaliella bardawil and Dunaliella salina. Int J Food Sci Nutr 58:373–382
Wang WJ, Liu XW, Gai XS, Ren JJ, Liu XF, Cai YL, Wang Q, Ren H (2015) Cucumissativus L. WAX2 plays a pivotal role in WAX biosynthesis, influencing pollen fertility and plant biotic and abiotic stress responses. Plant Cell Physiol 56:1339–1354
Watanabe S (1983) New and interesting green algae from soils of some Asian and Oceanian regions. Arch Protistenkd 127:223–270
Waterbury JB, Stanier RY (1981) Isolation and growth of cyanobacteria from marine and hypersaline environments. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes. Springer, Berlin, pp 221–223
Wu Z, Dejtisakdi W, Kermanee P, Ma C, Arirob W, Sathasivam R, Juntawong N (2017) Outdoor cultivation of Dunaliella salina KU 11 using brine and saline lake water with raceway ponds in northeastern Thailand. Biotechnol Appl Biochem 64:938–943
Zhukova NV, Aizdaicher NA (1995) Fatty acid composition of 15 species of marine microalgae. Phytochemistry 39:351–356
Acknowledgements
We would like to show our gratitude to Prof. Michael Borowitzka and the two reviewers for their perceptive comments that have greatly helped to improve this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salehipour-Bavarsad, F., Riahi, H., Hejazi, M.A. et al. Screening terrestrial and aquatic strains of Dunaliella from the eco-taxonomical perspective: a comparative study on fatty acid compositions as habitat indicators. J Appl Phycol 34, 461–474 (2022). https://doi.org/10.1007/s10811-021-02645-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02645-3