Abstract
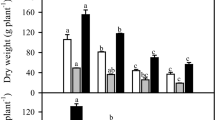
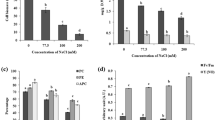
Maintaining K+/Na+ homeostasis and redox homeostasis is crucial for the tolerance of P. haitanensis to hypersalinity. However, the precise link between the signaling role of reactive oxygen species (ROS) and K+/Na+ homeostasis remains poorly characterized. In this study, we analyze hydrogen peroxide (H2O2) production and flux as well as H2O2 effects on K+, Na+, and Ca2+ transport in P. haitanensis under hypersaline condition. An exposure to hypersaline stress (110‰, 15 min) rapidly increased the H2O2 content and efflux in P. haitanensis cells, which was counteracted by the rapid increase in superoxide dismutase activity and activation of defense responses. The enhanced Na+ efflux and Ca2+ influx induced by salt stress were substantially suppressed by an NADPH oxidase inhibitor (DPI) or an ROS scavenger (DMTU). Additionally, Na+ efflux decreased in response to a plasma membrane Ca2+-permeable channel inhibitor (verapamil). This suggested that NADPH oxidase-mediated H2O2 production may promote Na+ efflux via the Ca2+-dependent Na+/H+ antiporter system in salt-stressed P. haitanensis thalli. Moreover, salt-induced H2O2 accumulation also enhanced K+ efflux, which was alleviated by exogenous Ca2+. H2O2 and Ca2+ may independently mediate K+ homeostasis in P. haitanensis. H2O2-induced K+ leakage may induce cells to switch from normal metabolic activities to those associated with adaptation and repair. The present results provide new insight for clarifying the relationship between salt-induced ROS signaling and ion homeostasis in intertidal seaweed species.







Similar content being viewed by others
References
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Blouin NA, Brodie JA, Grossman AC, Xu P, Brawley SH (2011) Porphyra: a marine crop shaped by stress. Trends Plant Sci 16:29–37
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257
Brawley SH, Blouin NA, Ficko-Blean E, Wheeler GL, Lohr M, Goodson HV, Jenkins JW, Blaby-Haas CE, Helliwell KE, Chan CX, Marriage TN, Bhattacharya D, Klein AS, Badis Y, Brodie J, Cao Y, Collén J, Dittami SM, Gachon CMM, Green BR, Karpowicz SJ, Kim JW, Kudahl UJ, Lin S, Michel G, Mittag M, Olson BJSC, Pangilinan JL, Peng Y, Qiu H, Shu S, Singer JT, Smith AG, Sprecher BN, Wagner V, Wang W, Wang ZY, Yan J, Yarish C, Zäuner-Riek S, Zhuang Y, Zou Y, Lindquist EA, Grimwood J, Barry KW, Rokhsar DS, Schmutz J, Stiller JW, Grossman AR, Prochnik SE (2017) Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc Nat Acad Sci USA 114:E6361–E6370
Cao M, Xu K, Yu X, Bi G, Mao Y (2020) A chromosome level genome assembly of P. haitanensis (Bangiales, Rhodophyta). Molec Ecol Resour 20:216–227
Chakraborty K, Bose J, Shabala L, Shabala S (2016) Difference in root K+ retention ability and reduced sensitivity of K+-permeable channels to reactive oxygen species confer differential salt tolerance in three brassica species. J Exp Bot 67:4611–4625
Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ. 28:1230–1246
Chen C, Ji D, Xie C, Xu Y, Liang Y, Zhen Y, Shi X, Wang F, Zhao L (2008) Preliminary study on selecting the high temperature resistance strains and economic traits of Porphyra haitanensis. Acta Oceanol Sinica (In Chinese). 5:100–106
Chen J, Wang WH, Wu FH, He EM, Liu X, Shangguan ZP, Zheng HL (2015) Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci Rep 5:12516
Chen T, Wang W, Xu K, Xu Y, Ji D, Chen C, Xie C (2019) K+ and Na+ transport contribute to K+/Na+ homeostasis in P. haitanensis under hypersaline stress. Algal Res 40:101526
Chung JS, Zhu JK, Bressan RA, Hasegawa PM, Shi HH (2008) Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J 53:554–565
Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci 123:1468–1479
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65:1259–1270
Drerup MM, Schlucking K, Hashimoto K, Manishankar P, Steinhorst L et al (2013) The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant 6:559–569
Flowers T (2004) Improving crop salt tolerance. J. Exp. Bot. 55:307–319
Henzler T, Steudle E (2000) Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot 51:2053–2066
Hossain MS, Dietz KJ (2016) Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front Plant Sci 7:548
Ji DH, Xu Y, Xiao HD, Chen CS, Xu K, Xie CT (2016) Superoxide dismutase genes in P. haitanensis: molecular cloning, characterization and mRNA expression. Acta Oceanol Sinica 35:101–111
Kumar M, Gupta V, Trivedi N, Kumari P, Bijo AJ, Reddy CRK, Jha B (2011) Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta). Environ Exp Bot 72:194–201
Li L, Kim BG, Cheong YH, Pandey GK, Luan S (2006) A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Nat Acad Sci USA. 103:12625–12630
Li J, Chen G, Wang X, Zhang Y, Jia H, Bi Y (2011) Glucose-6-phosphate dehydrogenase-dependent hydrogen peroxide production is involved in the regulation of plasma membrane H+-ATPase and Na+/H+ antiporter protein in salt-stressed callus from Carex moorcroftii. Physiol Plantarum 141:239–250
Lin KC, Jwo WS, Chandrika NNP, Wu TM, Lai MH, Wang CS, Hong CY (2016) A rice mutant defective in antioxidant-defense system and sodium homeostasis possesses increased sensitivity to salt stress. Biol Plantarum 60:86–94
Lu YJ, Li NY, Sun J, Hou PC, Jing XS Zhu HP, Deng SR, Han YS, Huang XX, Ma XJ, Zhao N, Zhang Y.H, Shen X, Chen SL (2012) Exogenous hydrogen peroxide, nitric oxide and calcium mediate root ion fluxes in two non-secretor mangrove species subjected to NaCl stress. Tree Physiol 33(1):81-95.
Lu X, Huan L, Gao S, He L, Wang G (2016) NADPH from the oxidative pentose phosphate pathway drives the operation of cyclic electron flow around photosystem I in high-intertidal macroalgae under severe salt stress. Physiol Plantarum 156:397–406
Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J Exp Bot 63:305–317
Mittler R, Vanderauwera S, Gollery M, Breusegem FV (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135:702–708
Niu LJ, Liao WB (2016) Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front Plant Sci 7:230
Pardo JM, Cubero B, Leidi EO, Quintero FJ (2006) Alkali cation exchangers: roles on cellular homeostasis and stress tolerance. J Exp Bot 57:1181–1199
Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406:731–734
Rejeb K, Abdelly C, Savouré A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Bioch 80:278–284
Samanta P, Shin S, Jang S, Kim JK (2019) Comparative assessment of salinity tolerance based on physiological and biochemical performances in Ulva australis and Pyropia yezoensis. Algal Res 42:101590
Sanchez-Barrena MJ, Martinez-Ripoll M, Zhu JK, Albert A (2005) The structure of the Arabidopsis thaliana SOS3: molecular mechanism of sensing calcium for salt stress response. J Mol Biol 345:1253–1264
Sathiyaraj G, Srinivasan S, Kim YJ, Lee OR, Parvin S, Balusamy RD, Khorolragchaa A, Yang DC (2014) Acclimation of hydrogen peroxide enhances salt tolerance by activating defense-related proteins in Panax ginseng CA Meyer. Mol Biol Rep 41:3761–3771
Schreiber U (2004) Pulse–amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: George, CP, Govindjee, (eds.) Chlorophyll a fluorescence: a signature of photosynthesis. Kluwer, Dordrecht pp 219–319.
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plantarum 133:651–669
Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141:1653–1665
Smirnoff N, Arnaud D (2018) Hydrogen peroxide metabolism and functions in plants. New Phytol 221:1197–1214
Sun J, Dai SX, Wang RG, Chen SL, Li NY, Zhou XY, Lu CF, Shen X, Zheng XJ, Hu ZM (2009) Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol 29:1175–1186
Sun JA, Li LS, Liu MQ, Wang MJ, Ding MQ, Deng SR, Lu CF, Zhou XY, Shen X, Zheng XJ (2010) Hydrogen peroxide and nitric oxide mediate K+/Na+ homeostasis and antioxidant defense in NaCl-stressed callus cells of two contrasting poplars. Plant Cell Tiss Org 103:205–215
Sung MS, Hsu YT, Hsu YT, Wu TM, Lee TM (2009) Hypersalinity and hydrogen peroxide upregulation of gene expression of antioxidant enzymes in Ulva fasciata against oxidative stress. Mar Biotechnol 11:199–209
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Verslues PE, Batelli G, Grillo S, Agius F, Kim YS, Zhu J, Agarwal M, Katiyar-Agarwal S, Zhu JK (2007) Interaction of SOS2 with nucleoside diphosphate kinase 2 and catalases reveals a point of connection between salt stress and H2O2 signaling in Arabidopsis thaliana. Mol Cell Biol 27:7771–7780
Wang WL, Li HQ, Lin XZ, Zhang F, Fang BS, Wang ZK (2016a) The effect of polar auxin transport on adventitious branch formation in Gracilaria lichenoides in vitro. Physiol Plantarum 158:356–365
Wang Y, Li XQ, Li JY, Bao Q, Zhang FC, Tulaxi G, Wang ZC (2016b) Salt-induced hydrogen peroxide is involved in modulation of antioxidant enzymes in cotton. Crop J 4:490–498
Wang H, Shabala L, Zhou M, Shabala S (2018) Hydrogen peroxide-induced root Ca2+ and K+ fluxes correlate with salt tolerance in cereals: towards the cell-based phenotyping. Int J Mol Sci 19:702
Wang W, Xu Y, Chen T, Xing L, Xu K, Xu Y, Ji D, Chen C, Xie C (2019) Regulatory mechanisms underlying the maintenance of homeostasis in P. haitanensis under hypersaline stress conditions. Sci Total Environ 662:168–179
Wang W, Chen T, Xu Y, Xu K, Xu Y, Ji D, Chen C, Xie C (2020) Investigating the mechanisms underlying the hyposaline tolerance of intertidal seaweed, P. haitanensis. Algal Res 47:101886
Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125:1347–1360
Yang YQ, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol 217:523–539
Zhang Y, Xie CT, Chen CS, Ji DH, Zhou WW (2011) Physiological responses of gametophytic blades of P. haitanensis to rising temperature stresses. J Fish China 35:379–386 (in Chinese)
Zheng HY, Wang WL, Xu K, Xu Y, Ji DH, Chen CS, Xie CT (2020) Ca2+ influences heat shock signal transduction in P. haitanensis. Aquaculture 516:734618
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No: 31872567 and 41806185), National Key R&D Program of China (Grant No: 2018YFD0900106 and 2018YFD0901500), Fujian Province Science and Technology Major Project (2019NZ08003), and “China Agriculture Research System (Grant No: CARS-50)”. We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
WW and LX conducted the data analysis and wrote the first draft of the manuscript. KX, DJ, and YX participated in the sample processing and data collection. CX and CC contributed to design and interpretation of results. All authors contributed to writing, revising, and approving the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare they do not have any conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, W., Xing, L., Xu, K. et al. Salt stress-induced H2O2 and Ca2+ mediate K+/Na+ homeostasis in Pyropia haitanensis. J Appl Phycol 32, 4199–4210 (2020). https://doi.org/10.1007/s10811-020-02284-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02284-0