Abstract
Herein we studied the efficiency of a protective transparent and reversible nano silicone wax coatings (NSW) to protect bronze artifacts extracted from Herakleon City, Abu Kir Bay, Alexandria, Egypt. The nano structured silicone wax film resulted in the formation of a structure that can act as a barrier layer from corrosion. The application of NSW coatings on bronze artifacts was evaluated by electrochemical impedance spectroscopy and scanning electron microscopy techniques. Exposure of bronze samples with and without the nano silicone wax coating to sodium chloride solution led to a remarkable inhibition of corrosion for the coated samples only. Comparison of the inhibition efficiency obtained from samples coated with nano silicone wax coatings to that obtained from silicone wax coatings in normal size revealed that the protection efficiency is very high in case of nano silicone wax coatings compared to the traditional silicone wax coatings. The application of nano silicone wax coatings on bronze artifacts can pave the way into the development of safe and tailored solutions in the field of ancient metal artifacts conservation.
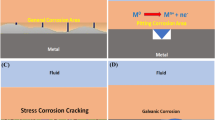
Graphical abstract

Similar content being viewed by others
1 Introduction
Conservation of historical and archaeological metallic artifacts from dangerous corrosion phenomena, that affects the physical and chemical stability of metals, is crucial due to the potential loss of the metallic artifact. More efforts are needed to find reliable conservation methods for the protection of metallic artifacts. The factors that determine the type of corrosion that attacks metals in the marine environment and its rate, can be classified into three factors: physical, chemical and biological factors. The most important key factors in influencing the rate of corrosion of metals in sea water are the dissolved oxygen content, temperature, flow rate, salinity, acidity and biological activity [1]. Bronze artifacts extracted from seawater suffer from its interaction with the most aggressive corrosion agent for copper-based alloys, chloride ion Cl−, forming cuprous chloride causing degradation process of the bronze alloy. The reactive cuprous chloride species induces further degradation of the bronze alloy, due to the interaction with oxygen and humidity from atmosphere forming the greenish atacamite Cu2(OH)3Cl which in turn converted to cuprite Cu2O due to further interaction with O2 and water causing disfiguration of the bronze artifacts. This process is called bronze disease [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. The common method for protection of metallic antiquities is the use of coating that consists of a passive polymer matrix either itself or loaded with chemically active corrosion inhibitors such as benzotriazole BTA or one of its derivatives [16,17,18,19,20]. These polymeric coatings have many disadvantages such as: distortion, presence of fine cracks and decreasing of compression strength [21]. Recently, application of nanomaterials and nanocomposites for the protection and conservation of metallic antiquities attracted the attention of researchers due to their acceptable physical and chemical stability in addition to their antibiological activity [22,23,24,25,26,27,28,29,30]. The aim of this study is to apply nano silicon wax (NSW) coating on bronze artifacts extracted from Abu Kir Bay, Alexandria, Egypt. The bronze sample of this study (Fig. 1), is food ladle from the set discovered in Heraklion city in Abu Kir Bay, Egypt, by the European which started in 1996 until 2002.
During this study, evaluation of (NSW) as protective coating for bronze artifacts by electrochemical impedance spectroscopy technique (EIS) will be carried out. Comparison between the efficiency of (NSW) as protective coating for bronze alloy and (SW) in its original particle size will be carried out and discussed.
2 Materials and methods
2.1 Preparation of nanosilicon wax
Chemicals used in this study were purchased from Sigma Chemical Company. Poly dimethyl siloxane PDMS (silicon wax) was prepared in nano particles according to the procedure previously published by Piskoti [31]. The chemical formula for PDMS is CH3[Si(CH3)2O]nSi(CH3)3, where n is the number of repeating monomer [SiO(CH3)2] units. PDMS chemical structure is given in Fig. 2. The silicon wax prepared was characterized by transmission electron microscope (TEM), JEM-1230 Electron Microscope Operated at 60 kV (JEOL Ltd., Tokyo, Japan), Fig. 3. The particle size of NSW prepared was ranged from 17 to 32 nm as shown in the figure. The bronze samples used in this study, were washed with freshly prepared double distilled water followed by ethanol and then dried in air prior to coating. The coatings were applied to the bronze samples by immersion of the bronze panels in a 500 mL beaker containing the required coating material. The excess coat was drained. The coated bronze panels were allowed to dry, at room temperature (20–25 °C), by hanging them perpendicularly until a visually dry surface was obtained (≈ 30 min). The process of dipping and drying was repeated for each sample 2–3 times in order to obtain a constant coat thickness of approximately 60 ± 5 µm. The coat thickness was measured using a coating thickness gauge, Minitest 300FN, Elektrophysik, Erichsen Testing Equipment [32].
The criteria used to evaluate nano silicon wax as a conservative material for bronze artifacts was the impedance spectroscopy (EIS), scanning electron microscopy (SEM) scans and calculating the degree of surface coverage by the (NSW) material.
2.2 Electrochemical measurements
All electrochemical experiments were performed in aqueous solution of 3.5% sodium chloride. All experiments were performed at room temperature and relative humidity RH between 55 and 60% simulating the coastal environment in which the antiquates were found. Electrochemical studies were carried out in a three electrode system with bronze samples as working electrodes, platinum sheet was used as counter electrode and Calomel electrode (SCE) was used as the reference electrode. The working electrode was constructed from bronze alloy with the same chemical composition of the bronze food ladle under study. The bronze samples were constructed with dimensions 5 cm × 5 cm × 0.5 mm with exposed area to solution 29 cm2. ACM 604 Potentiostate instrument was used for the electrochemical impedance measurements with frequency range 0.01 to 104 Hz. Analysis of the impedance spectra was performed by Zisimpwin 3.2 program by fitting the experimental data to the appropriate equivalent circuit.
2.3 Characterization of insulating coats
The morphologies of the insulating coats on bronze samples were investigated by Scanning Electron Microscopy (SEM) technique using JOEL instrument. Scanning electron microscopy (SEM) were performed on films deposited on OTEs with a JEOL T 330 microscope fitted with a Tracor Northern Microanalysis Addessory Electrodes, sputter-coated with a thin gold film before (SEM) was carried out (magnification ×1500 at 20 kV).
2.4 Characterization and preparation of bronze samples
Bronze samples were prepared in a way to stimulate archaeological artifacts; for its wealth wasting to carry out un-granted result tests on archeological artefacts. The bronze samples used in this study was analyzed using the scanning electron microscopy technique (SEM) accompanied with (EDX) unit for elemental analysis. The (EDX) spectrum is presented in Fig. 4. The chemical composition of the bronze alloy used in this study was found to be (% wt): Cu2+ 88; Sn 11.8; Fe 0.01; Pb 0.01.
2.5 Surface analysis
X-ray diffraction (XRD) technique was used to know the chemical composition of the corrosion products formed on the bronze antiquit under study. Three samples were taken from different positions of the food ladle and analyzed using X-ray Powder Diffraction XRD-phaser-Bruker-Germany D2 Philips PW3710/31 Diffractometer. Analysis of samples using (XRD) technique reveals the presence of cuprite Cu2O, Cuprous chloride (atacamite) Cu2Cl2 and basic cupric chloride (paratacamite) Cu2(OH)3Cl. This is caused by the aggressive attack of the chloride ion Cl− present in sea water to the copper alloy of the bronze antiquites. Analysis of bronze samples using (SEM) technique reveals that the bronze samples is surrounded by different corrosion products Fig. 5.
3 Results and discussion
3.1 Electrochemical impedance spectroscopy EIS technique
Electrochemical impedance spectroscopy (EIS) technique is the most widely electrochemical method used for the assessment of metal coatings because, it is accurate and non-destructive technique for both the metal and the coating material. EIS is used in this study to evaluate the efficiency of nano silicon wax as insulating material to protect bronze ladle extracted from Abu Kir Bay, Alexandria, Egypt.
Blank bronze samples with exposed surface area 29 cm2 have been prepared in addition to bronze samples coated with the nano silicon wax which, were prepared with concentrations 3% and 7%, respectively. The impedance spectra were measured in 3.5% sodium chloride solution as a simulation for the salinity of sea water in the coastal area were the bronze antiquates found. The impedance measurements were repeated periodically for 6 months.
Figure 6, represents the nyquist plot for the blank bronze sample in presence of 3.5% NaCl solution.
The figure represent capacitive behavior indicating the presence of the charge transfer resistance associated with the effect of ionic double-layer capacitance. The impedance spectra for Nyquist plot was analyzed by fitting the experimental data using Zsimpwin program to a simple equivalent circuit model (Fig. 7). The equivalent circuit represents the chemical reactions that might takes place at the interface between the bronze sample, represented by the patina formed on the bronze sample which is composed of cuprite (Cu2O), and the NaCl solution.
The equivalent circuit model includes the solution resistance Rs and the constant phase element Q which is placed in parallel to charge transfer resistance element Rct. The Q is used normally to replace the capacitor in the equivalent circuit to express the non-homogeneity in the system and is defined by two values, Q and n, where 0 ≤ n ≤ 1. In case of n = 1, the Q values is replaced by the capacitance C. The values of the electrochemical parameters obtained from EIS for bronze sample in 3.5% NaCl are given in Table 1.
Inspection of the data given in Table 1 reveals that the solution resistance Rs is high. This can be due to the high concentration of NaCl test solution. The charge transfer resistance Rct is high indicating the nobility of the bronze samples.
3.2 Nano silicon wax insulating material
Silicone waxes are well-known as Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer due to its versatility and properties leading to many applications. It is particularly known for its unusual rheological (or flow) properties. PDMS is optically clear and, in general is inert, non-toxic and non-flammable. It is one of several types of silicone oil (polymerized siloxane) [33]. Figures 8 and 10, represent nyquist plots while Figs. 9 and 11, represent bode plots, obtained for bronze samples coated with 3% and 7% NSW as insulating material for bronze antiquites, measured in 3.5% NaCl solution.
Figures 8 and 10, represent capacitive behavior indicating the presence of the charge transfer resistance associated with the effect of ionic double-layer capacitance. It could be noticed that during the first month of exposure for bronze coated with 3% NSW, the nyquist plot in Fig. 8a is associated with a diffusion tail, indicating that the insulating material still not compact giving the chance for the penetration of the ions from the solution to diffuse through the interface between the solution and the coated bronze. This diffusion tail is disappeared during the next month of exposure until the end of the 6 months of exposure as shown in Fig. 8b. This can be explained by assuming that the silicon wax nano particles have the opportunity to arrange themselves in a way to form compact layer on the bronze samples. This behavior is one of the advantages of the nanotechnology. Consequently, the inhibition efficiency of the NSW increased with time of exposure. The impedance spectra for Nyquist plots of 3% and 7% NSW, were analyzed by fitting the experimental data using Zsimpwin program to a simple equivalent circuit model (Fig. 7). The equivalent circuit represents the chemical reactions that might take place at the interface between the bronze sample coated with 3% NSW and the NaCl solution.
The equivalent circuit model used is described before in discussing the impedance behavior of bronze samples without coating. The electrochemical impedance parameters and the protection efficiency for bronze samples coated with 3% and 7% NSW are given in Tables 2 and 3. The protection efficiency is calculated from the following equation:
where RCT0 is the charge transfer resistances in the uncoated bronze and RCT is the charge transfer resistance RCT of the coated bronze.
It is clear from the data in Tables 2 and 3, that the charge transfer resistance Rct and the inhibition efficiency of the 3% and 7% NSW coating material increase with exposure time reaching 99.1% for 3% NSW and 99.7% for 7% NSW, after 6 months of exposure. This is in accordance of the explanation that previously showed and assigned as advantage of using the silicon wax in nano particles.
Figures 9 and 11, represent the bode plots obtained from impedance measurements of 3% and7% NSW, respectively. Inspection of the graphs showd that the impedance response increases with the exposure time.
Figure 12, represent the relation between the inhibition efficiency of the bronze samples coated by NSW insulation materials 3% and 7%, respectively. It could be noticed from the graph that the inhibition efficiency of both concentrations 3% and 7% are similar to each other's. This means that the percentage of NSW insulating material has no significant effect on the inhibition efficiency. In the meantime, the inhibition efficiency last at 99% for 6 months of exposure indicating the durability of NSW coatings and its worth using as insulating material for bronze artifacts.
3.3 Scanning electron microscopy SEM
Scanning electron microscopy (SEM) is characterized by giving a superficial vision by magnifying the surface to many thousands times. SEM was used in this study to shed the light on the importance of surface analysis and its agreement with the electrochemical measurements. Figure 13, represents the SEM microphotography for the bronze sample without insulating material. Figure 14, represents the SEM micrographs for the bronze sample coated with NSW insulating material.
The SEM micrograph for the blank sample represents the formation of block like structure representing the cuprite layer formed on the bronze sample. The grooves in the cuprite layer indicated that the cuprite layer is not protective.
The SEM micrographs represented in Fig. 14, showed that the layers formed either in presence of 3% and 7% NSW coating material, covers completely all the bronze surface and the oxide layer formed on it without any defects in the surface. It can be noticed that after 6 months of exposure the layers formed appears to be more smooth and compact indicating that the efficiency of NSW coatings increased with increasing the exposure time. It can be interpreted by considering that the nano silicon particles rearrange themselves on the bronze surface with time and become more compact and fill even the tiny gaps in the insulating material. Also, there is no significant difference observed between 3 and 7% NSW concentrations. These results are in accordance with the results obtained from impedance measurements.
3.4 Durability of the insulating materials
Durability of the insulating material is its ability to maintain the required performance over a long time, under the foreseeable actions. Lack of durability of insulating material can lead to its failure or at least its deterioration with time [34]. Therefore, the long-term performance of insulating material needs to be understood in a quantitative term. Sustaining the long-term performance of these insulating materials requires understanding the deterioration of the insulating materials over time. Calculating the degree of surface coverage is then very crucial to understand the deterioration behavior of the insulating materials under study. Table 4, represents the degree of surface coverage of the insulating materials over the time until 180 days.
Degree of surface coverage Ө of the bronze samples coated by nano silicon wax can be calculated from the following equation:
where Ө is the degree of surface coverage, IE is the inhibition efficiency.
Inspection of the data in Table 4 and the relations described in Fig. 15 reveals that the NSW insulating material used starts with a fairly large degree of surface coverage 0.92 for 3% NSW and 0.86 for 7% NSW at the beginning; increasing with exposure time and getting close to one gradually after long time of exposure (6 months). This fact can be explained by considering that not only the NSW can act as corrosion inhibitor for bronze artifacts but also they rearrange themselves with time in a way allow the particles to cover nearly all the surface. We are not sure that there is a study relate the degree of deterioration during laboratory experiments to that occur in the real environmental conditions. We suggest that the resistance to deterioration in the laboratory for 6 months means that the antiquities coated with this insulating material can resist its deterioration for years. It is clear that the concentration variation between 3 and 7% did not give significant effect on the degree of surface coverage, as they reach similar degree of protection with time. These results agree with the observation got from both the electrochemical measurements and the scanning electron microscopy techniques.
In our previous work, we studied silicon wax coating, in its normal particle size, as insulating material to protect bronze artifacts from corrosion. Silicon wax in its normal particle size starts with very high inhibition efficiency, 98% at the early beginning of exposure to saline solution and decreases slightly with time reaching 95.5% after 6 months. On the contrary, the inhibition efficiency of nano silicon wax increased with time till 6 months and the graph still increasing. Figure 16, shows a comparison between silicon wax and nano silicon wax coatings as insulating materials for bronze alloy.
We can conclude from the data shown in graph that silicon wax in both normal particle size and nano-particle size, can be used as insulating material for bronze artifacts to protect them from corrosion. There is a main effect of nano-sized silicon wax, is that the inhibition efficiency in presence of nano particles increased with exposure time until 6 months and promising to increase more even after the 6 months of exposure. These results are confirmed by the results obtained from impedance measurements and the micrographs obtained by scanning electron microscope. This fact leads to the conclusion that silicon wax can be used as insulating material for corrosion protection of bronze samples but using silicon wax in its nano-sized particles is perfect for this purpose.
4 Conclusions
Nano silicon wax insulating material has considerable protective behavior for bronze alloy. The inhibition efficiency is very high reaches 99% after exposure of 6 months. There is no significant difference in inhibition efficiency between 3 and 7% active ingredients in the insulating materials indicating that the ratio of the active ingredients in the insulating materials is not controlling factor. The scanning electron microscopy micrographs showed that the film formed is perfect with no defects during 6 months 'of exposure. The sustainability of the inhibition efficiency in presence of silicon wax over the 6 months can be explained by the formation of multilayers on the bronze samples. The observations of the SEM is in good agreement with the data obtained from EIS measurements. The degree of surface coverage is an indicative of the durability of the insulating material. Silicon wax did not lose its durability over the 6 months.
References
Durodola BM, Olugbuyiro JAO, Moshood SA, Fayomi OS, Popoola API (2011) Study of influence of zinc plated mild steel deterioration in seawater environment. Int J Electrochem Sci 6(11):5605–5616
Faraldi F, Cortese B, Caschera D, Di Carlo G, Riccucci C, de Caro T, Ingo GM (2017) Smart conservation methodology for the preservation of copper-based objects against the hazardous corrosion. Thin Solid Films 622:130–135
Mezzi A, de Caro T, Riccucci C, Faraldi F, Veroli C, Caschera D (2013) Unusual surface degradation products grown on archaeological bronze artefacts. Appl Phys A 113:1121–1128
Fink CG (1948) The corrosion handbook. Wiley, New York
Walker R (1980) Corrosion and preservation of bronze artifacts. J Chem Educ 57:277–280
Macleod ID (1981) Bronze disease: an electrochemical explanation. Bull ICCM 7:16–26
Robbiola L, Blengino JM, Fiaud C (1998) Morphology and mechanisms formation of natural patinas on archaeological Cu-Sn alloys. Corros Sci 40:2083–2111
Scott DA (2002) Copper and bronze in art, corrosion, colorants, conservation. Paul Getty Conservation Institute, Malibu
Scott DA (1985) Periodic corrosion phenomena in Bronze antiquities. Stud Conserv 30:49–57
Dillman P, Beranger G, Piccardo P, Matthiessen H (2007) Corrosion of metallic heritage artefacts: investigation, conservation and prediction for long-term behavior. Woodhead Publishing, Sawston
Scott DA (1994) Bronze disease: a review of some chemical problems and the role of relative humidity. JAIC 33:1–23
Mattsson E, Nord AG, Tronner K, Fjaestad M, Lagerlof A, Ullen I, Borg GC (1996) Deterioration of archaeological material in soil. In: Central Board of National Antiquities (ed) Rik, vol 10. Stockholm, p 50
Taylecote RF (1979) The effect of soil conditions on the long-term corrosion of buried tinbronzes and copper. J Archaeol Sci 6:345–368
Nord AG, Mattsson E, Tronner K (2005) Factors influencing the long-term corrosion of bronze artefacts in soil. Prot Met 41:309–316
Scott DA (1990) Bronze disease: a review of some chemical problems and the role of relative humidity. JAIC 29:193–206
Giuliania C, Pascuccia M, Riccuccia C, Messinaa E, Salzano de Lunab M, Lavorgnab M, Ingoa GM, Di Carloa G (2018) Chitosan-based coatings for corrosion protection of copper-based alloys: a promising more sustainable approach for cultural heritage applications. Prog Org Coat 122:138–146
Watkinson D (2010) Shreir’s corrosion. In: Preservation of metallic cultural heritage, vol 4, 4th edn. Elsevier, London, pp 3307–3340
Finšgar M, Milošev I (2010) Inhibition of copper corrosion by 1,2,3-benzotriazole: a review. Corros Sci 52:2737–2749
Pillard DA, Cornell JS, Dufresne DL, Hernandez MT (2001) Toxicity of benzotriazole and benzotriazole derivatives to three aquatic species. Water Res 35:557–560
Health Council of the Netherlands: Dutch expert committee on occupational standards (DECOS). 1,2,3-Benzotriazole. The Hague: Health Council of the Netherlands, 2000; publication no. 2000/14OSH. ISBN: 90-5549-348-1
Francis LF, Mccormick AV, Vaessen DM, Payne JA (2002) Development and measurement of stress in polymer coatings. J Mater Sci 37:4717–4731
Fedel M (2009) Environmentally friendly hybrid coatings for corrosion protection: saline based pre—treat-menrs and nano structured waterborne coatings, Department of materials engineering and Industrial technologies, Ph. D Thessis, University of Torento, Italy, 7–10
Joseph R, Dorothy R (2013) Corrosion resistance of nano-particle incorporated nano coatings. Eur Chem Bull 2(12):965–970
Langrodi E (2009) Synthesis and characterization of nanosilica based coatings for protection of antique articles. Int J Nanotechnol 6(10):10–11
Pathak S, Khanna A (2012) Sol–gel nanocoating for corrosion protection, corrosion protection and control. Wood Head Publishing Limited, New Delhi
Riad M, Harhash A, Elhiny O, Salem G (2015) Evaluation of the shear bond strength of orthodontic adhesive system containing antimicrobial silver nanoparticles on bonding of metal brackets to Enamel. Life Sci J 12(59):32–35
Tsirita K (2012) The selection of the gap-filling material for the conservation of a Chinese Shang dynasty bronze vase, Technological Educational Institute of Athens, Department of conservation of antiquities and works of art. Vienna Congress, the decorative conservation and the applied arts
Vassiliou P (2008) Copper alloy and silver artifacts protection by coating with nano- alumina pigments, School of chemical Engineering, National Technical University of Athens, pp 132–137
Wang J, Wu Y, Zhang S (2014) A new coating system modified with nano-sized particles for archaeological bronze protection. Stud Conserv 59(4):268–275
Yan X, Xu G (2010) Corrosion and mechanical properties of epoxy- polyurethane/bronze composite coating with low infrared emissivity, College of material science and technology, Nanjing university of Aero-nautics and Astronautics, China
Piskoti C (2010) US, Silicone wax emulsion and method of manufacture, US20100210491A1
Abdel-Gaber AM, Abd-El Nabey BA, Khamisa E, Abdelattef OA, Aglan H, Ludwick A (2010) Influence of natural inhibitor, pigment and extender on corrosion of polymer coated steel. Prog Org Coat 69:402–409
Wolf MP, Salieb-Beugelaar GB, Hunziker P (2018) PDMS functionalities-properties, modifications strategies, and applications. Prog Polym Sci 83:97–134
Ramanauskas J, Stankevicius V (1998) Weather durability of external wall thermal insulation system with a thin layer plaster finish. Statyba 4(3):206–213
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdullatef, O.A., Helal, M.A. & Anwar, S.M.M. Innovative nano silicone wax coatings for the conservation of bronze artifacts from corrosion. J Appl Electrochem 52, 1121–1131 (2022). https://doi.org/10.1007/s10800-022-01690-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01690-1