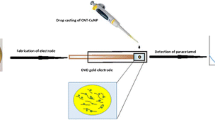
Abstract
Dimenhydrinate (DMH) is a salt composed by the combination of two active pharmaceutical ingredients: diphenhydramine (DIP) and 8-chlorotheophylline (CTP). In this work, the glassy carbon electrode was electrochemically deposited in two steps. The electrode was first inserted in the Alizarin Yellow R (AYR) solution and it was placed on the surface of the electrode after 8 scans. In order to modify the electrode with copper nanoparticles, the prepared electrode was first inserted into the copper sulfate solution and coated with copper nanoparticles using cyclic voltammetry CuNps/PAYR/GCE was fabricated. SEM images showed that the copper nanoparticles were deposited on the electrode. The electrocatalytical effects of the electrochemical sensor were also studied by cyclic voltammetry (CV) and differential pulse voltammetry (DPV) techniques. This electrode was used for detection simultaneously of DIP (OX1) and CTP (OX2) in pharmaceutical samples. The results showed that the highest sensitivity for DMH was obtained at pH = 10. Finally, Also, the detection of limit, sensitivity and linear range were calculated as 0. 29 µM, 0.043 and 1–3000 µM respectively. CuNps/PAYR/GCE presented considerable advantages for instance high sensitivity, used for real samples, simple preparation, low detection of limit, and specially the simultaneous oxidation of CTP and DIP for detection of DMH.
Graphical abstract








Similar content being viewed by others
References
Ozkan CK, Tasdemir U, Tas C, Savaser A, Erol H, Ozkan Y (2013) Determination of dimenhydrinate nasal delivery system in the blood by RP-LC. Chromatographia 76:1521–1525
Halpert AG, Olmstead MC, Beninger RJ (2002) Mechanisms and abuse liability of the anti-histamine dimenhydrinate. Neurosci Biobehav R 26:61–67
Shah PB, Patel PU (2012) Q-absorbance ratio spectrophotometric method for the simultaneous estimation of cinnarizine and DMH in their combined dosage form. J Pharm Sci Bio-scientific Res 2(2):83–87
Lamie NT, Yehia AM (2015) Development of normalized spectra manipulating spectrophotometric methods for simultaneous determination of DMH and cinnarizine binary mixture. Spectrochim Acta A 150:142–150
Shah PB, Patel PU (2015) Q-absorbance ratio spectrophotometric method for the simultaneous estimation of cinnarizine and DMH in their combined dosage form. J Pharm Sci Bio-scientific Res 2(2):83–87
Lamie NT, Yehia AM (2015) Development of normalized spectra manipulating spectrophotometric methods for simultaneous determination of DMH and cinnarizine binary mixture. Spectrochim Acta A150:142–150
Vlassa M, Filip M, Pascalau V, Comanv DC (2009) Determination of purine derivatives in bovine urine using rapid chromatographic techniques. Archiva Zootechnica 12(4):59–70
El-Kafrawy DS, Belal TS (2016) Validated HPTLC method for the simultaneous determination of cinnarizine and DMH in their combined dosage form. J Assoc Arab U Basic Appl Sci 19(1):15–22
Ahmed AB, Abdelwahab NS, Abdelrahman MM, Salama FM (2017) Simultaneous determination of DMH, Cinnarizine and Cinnarizine impurity by TLC and HPLC chromatographic methods. B Faculty Pharm, Cairo U 55(1):163–169
Belal TS, Abdel-Hay KM, Clark CR (2016) Selective determination of DMH in presence of six of its related substances and potential impurities using a direct GC/MS method. J adv Res 7(1):53–58
Verdugo D, Cancilla M, Ge X, Gray N, Chang Y, Schultz P, Bertozzi C (2001) Discovery of estrogen sulfotransferase inhibitors from a purine library screen. J Med Chem 44(17):2683–2686
Shubietah RM, Zuhri AZA, Fogg AG (1999) Adsorptive stripping voltammetric determination of DMH at a hanging mercury drop electrode. Microchim Acta 130(3):165–171
Freitas JM, Costa Oliveira T, Silva PL, Gimenes DT, Abarza Munoz RA, Richter EM (2014) Development of a simple and fast electrochemical method for screening and stoichiometric determination of DMH. Electroanalysis 26(9):1905–1911
Rabenstein DL, Yamashita GT (1989) Determination of homocysteine, penicillamine, and their symmetrical and mixed disulfides by liquid chromatography with electrochemical detection. Anal Biochem 180:259–263
Vandeberg PJ, Johnson DC (1993) Pulsed electrochemical detection of cysteine, cystine, methionine, and glutathione at gold electrodes following their separation by liquid chromatography. Anal Chem 65:2713–2718
Rosie NT, Chelfi T, Hach B, Massai HBB, Laural NC (2010) Immobilization of Organic compounds on a Modified Electrode: The Electrochemical Sensor Route. Bullet Catal Soc Ind 9:68–73
Riquelme MA, Lucero MA, Villagrán M, Arévalo MC, Hernández-Creus HJ, Velez M, Aguirre J, Arce R, Ramírez G (2012) Glassy carbon modified electrode: polymer and supramolecular assembly of Co (II)- [tetra (O-aminophenyl) porphyrin] new material for electrocatalytic assays. Int J Electrochem Sci 7:9738–9747
Atta NF, Galal A, Azab SM (2012) Gold nanoparticles modified electrode for the determination of an antihypertensive drug. Electroanalysis 24:1431–1440
Filho OF, Dockal ER, Junior LHM, Teixeir MFS (2007) Electrochemical modified electrodes based on metal salen complexes. Anal Lett 40:1825–1852
Mani V, Vilian AT, Chen ES (2012) Graphene oxide dispersed carbon nanotube and iron phthalocyanine compositemodified electrode for the electrocatalytic determination of hydrazine. Int J Electrochem Sci 7:12774–12785
Amini N, Shamsipur M, Gholivand MB, Barati A (2017) A glassy carbon electrode modified with carbon quantum dots and polyalizarin yellow R dyes for enhanced electrocatalytic oxidation and nanomolar detection of L-cysteine. Microchem J 131:9–14
Chen CX, GaoY H (2007) Electrosynthesis of poly (neutral red) incorporated with ferrocenesulfonic acid. Electrochim Acta 52:7322–7329
Zhou T, Qin Y, Xu J, Tao YX, Lu MH, Kong Y (2015) Zinc ions doped poly(aniline-co-m-aminophenol) for high-performance supercapacitor. Synth Met 199:169–173
Doblhofer K (1980) Electrodes covered with thin, permeable polymer films. Electrochim Acta 25:871
Xin-Gui L, Qiu-Feng L (2008) Mei-Rong H (2008) Self-stabilized nanoparticles of intrinsically conducting copolymers from 5-sulfonic-2-anisidine. Small 41(41):1201–1209
Xin-Gui L, Mei-Rong H, Wei D, Yu-Liang Y (2002) Novel multifunctional polymers from aromatic diamines by oxidative polymerizations. Chem Rev 102:2925–3030
Mu SL, Zhang Y, Zhai JP (2009) The electrochemical copolymerization of aniline with 2,4-diaminophenol and the electric properties of the resulting copolymer. Electrochim Acta 54:3923–3929
Williams TR, Lautenschleger M (1963) Titration of weak acids with tetramethylgnanidine as solvent. Talanta 10:804–808
Zhang K, Zhang N, Zhang L, Xu J, Wang H, Wang C, Geng T (2011) Amperometric sensing of hydrogen peroxide using a glassy cabon electode modified with silver nanoparticles on poly (alizarin yellow R). Mikrochim Acta 173:135–141
Zhou Y, Tang W, Wang J, Zhang G, Chai S, Zhang L, Liu T (2014) Selective determination of dopamine and uric acid using electrochemical sensor based on poly (alizarin yellow R) film-modified electrode. Anal Methods 6(10):3437–3481
Liu A, Dong W, Liu E, Tang W, Zhu J, Han J (2010) Non-enzymatic hydrogen peroxide detection using gold nanoclusters-modified phosphorus incorporated tetrahedral amorphous carbon electrodes. Electrochim Acta 55:1971–1977
Huang J, Wang D, Hou H, You T (2008) Electrospun palladium nanoparticle-loaded carbon nanofibers and their electrocatalytic activities towards hydrogen peroxide and NADH. Adv Funct Mater 18:441–448
Cao D, Sun L, Wang G, Lv Y, Zhang M (2008) Kinetics of hydrogen peroxide electroreduction on Pd nanoparticles in acidic medium. J Electroanal Chem 621:31–37
Qiu R, Cha HG, Noh HB, Shim YB, Zhang XL, Qiao R, Zhang D, KimY Il, Pal U, Kang YS (2009) Preparation of dendritic copper nanostructures and their characterization for electroreduction. J Phys Chem C 113:15891–15896
Jamal M, Hasan M, Mathewson A, Razeeb KM (2012) Non-enzymatic and highly sensitive H2O2 sensor based on Pd nanoparticle modified gold nanowire array electrode. J Electrochem Soc 159:B825–B829
Shah PB, Patel PU (2012) Q-absorbance ratio spectrophotometric method for the simultaneous estimation of cinnarizine and dimenhydrinate in their combined dosage form. J Pharm Sci Bio-scientific Res 2(2):83–87
Özkan CK, Taşdemir U, Taş C, Savaşer A, Erol H, Özkan Y (2013) Determination of dimenhydrinate nasal delivery system in the blood by RP-LC. Chromatographia 76:1521–1525
El-Kafrawy DS, Belal TS (2016) Validated HPTLC method for the simultaneous determination of cinnarizine and dimenhydrinate in their combined dosage form. J Assoc Arab U Basic Appl Sci 19(1):15–22
Lamie NT, Yehia AM (2015) Development of normalized spectra manipulating spectrophotometric methods for simultaneous determination of dimenhydrinate and cinnarizine binary mixture. Spectrochim Acta A 150:142–150
Ahmed AB, Abdelwahab NS, Abdelrahman MM, Salama FM (2017) Simultaneous determination of Dimenhydrinate, Cinnarizine and Cinnarizine impurity by TLC and HPLC chromatographic methods. B Faculty Pharm Cairo U 55(1):163–169
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amini, N., Abdolahi, S.S., Naderi, K. et al. Construction of a sensitive electrochemical sensor for diphenhydramine and 8-chlorotophylline as a dimenhydrinate drug based on copper nanoparticles and polyalizarin yellow at two applied potentials. J Appl Electrochem 52, 617–626 (2022). https://doi.org/10.1007/s10800-021-01657-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01657-8