Abstract
Purpose
Pterygium is a hyaline degenerative disease of the conjunctiva characterized by the progression of fibrovascular connective tissue from the bulbar conjunctiva to the cornea. The mechanism of pterygium formation is still not fully understood. Transient receptor potential (TRP) channels are a group of ion channels with distinct characteristics. Recent indications suggest TRP channels may play a significant regulatory role in pterygium development, but previous studies have mainly focused on in silico analysis. Accordingly, in the present study, we aimed to decipher the expression signatures and role of TRP channels in pterygium development.
Methods
The study encompassed a cohort of 45 patients matched for age and gender distribution, comprising 30 individuals with primary pterygium (PP) and 15 individuals with recurrent pterygium (RP). The control group consisted of unaffected conjunctival tissue obtained from the same set of patients. High-throughput screening of differentially expressed TRP channels in pterygium tissues was achieved with the help of Fluidigm 96.96 Dynamic Array Expression Chip and reactions were held in BioMark™ HD System Real-Time PCR platform.
Results
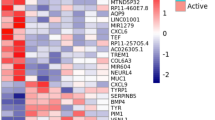
Statistically significant increases were found in the expression of 21 genes, mainly TRPA1 (p = 0.021), TRPC2 (p = 0.001), and TRPM8 (p = 0.003), in patients with PP, and in TRPC5 (p = 0.05), TRPM2 (p = 0.029), TRPM4 (p = 0.03), TRPM6 (p = 0.045), TRPM8 (p = 0.038), TRPV1 (p = 0.01) and TRPV4 (p = 0.025) genes in RP tissues.
Conclusion
Collectively, TRP channel proteins appear to play pivotal roles in both the development and progression of pterygium, making them promising candidates for future therapeutic interventions in patients afflicted by this condition.

Similar content being viewed by others
Data Availability
Not applicable.
References
Shahraki T, Arabi A, Feizi S (2021) Pterygium: an update on pathophysiology, clinical features, and management. Ther Adv Ophthalmol 13:25158414211020150
Shiroma H, Higa A, Sawaguchi S, Iwase A, Tomidokoro A, Amano S, Araie M (2009) Prevalence and risk factors of pterygium in a southwestern island of Japan: the Kumejima Study. Am J Ophthalmol 148:766-771.e761. https://doi.org/10.1016/j.ajo.2009.06.006
McCarty CA, Fu CL, Taylor HR (2000) Epidemiology of pterygium in Victoria, Australia. Br J Ophthalmol 84:289–292. https://doi.org/10.1136/bjo.84.3.289
Wang IJ, Hu FR, Chen PJ, Lin CT (2000) Mechanism of abnormal elastin gene expression in the pinguecular part of pterygia. Am J Pathol 157:1269–1276. https://doi.org/10.1016/s0002-9440(10)64642-1
Hu Q, Qiao Y, Nie X, Cheng X, Ma Y (2014) Bevacizumab in the treatment of pterygium: a meta-analysis. Cornea 33:154–160. https://doi.org/10.1097/ico.0000000000000037
Tsai YY, Chiang CC, Yeh KT, Lee H, Cheng YW (2010) Effect of TIMP-1 and MMP in pterygium invasion. Invest Ophthalmol Vis Sci 51:3462–3467. https://doi.org/10.1167/iovs.09-4921
Liang K, Jiang Z, Ding BQ, Cheng P, Huang DK, Tao LM (2011) Expression of cell proliferation and apoptosis biomarkers in pterygia and normal conjunctiva. Mol Vis 17:1687–1693
Ang LP, Chua JL, Tan DT (2007) Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol 18:308–313. https://doi.org/10.1097/ICU.0b013e3281a7ecbb
Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524. https://doi.org/10.1038/nature02196
Devi S, Kedlaya R, Maddodi N, Bhat KM, Weber CS, Valdivia H, Setaluri V (2009) Calcium homeostasis in human melanocytes: role of transient receptor potential melastatin 1 (TRPM1) and its regulation by ultraviolet light. Am J Physiol Cell Physiol 297:C679-687. https://doi.org/10.1152/ajpcell.00092.2009
Masumoto K, Tsukimoto M, Kojima S (2013) Role of TRPM2 and TRPV1 cation channels in cellular responses to radiation-induced DNA damage. Biochem Biophys Acta 1830:3382–3390. https://doi.org/10.1016/j.bbagen.2013.02.020
Tsai YY, Cheng YW, Lee H, Tsai FJ, Tseng SH, Lin CL, Chang KC (2005) Oxidative DNA damage in pterygium. Mol Vis 11:71–75
Shen A, Ye Y, Wang X, Chen C, Zhang H, Hu J (2005) Raman scattering properties of human pterygium tissue. J Biomed Opt 10:024036. https://doi.org/10.1117/1.1888345
Chen SJ, Zhang W, Tong Q, Conrad K, Hirschler-Laszkiewicz I, Bayerl M, Kim JK, Cheung JY, Miller BA (2013) Role of TRPM2 in cell proliferation and susceptibility to oxidative stress. Am J Physiol Cell Physiol 304:C548-560. https://doi.org/10.1152/ajpcell.00069.2012
Nazıroğlu M, Dikici DM, Dursun S (2012) Role of oxidative stress and Ca2+ signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res 37:2065–2075. https://doi.org/10.1007/s11064-012-0850-x
Gao H, Chen X, Du X, Guan B, Liu Y, Zhang H (2011) EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium 50:559–568. https://doi.org/10.1016/j.ceca.2011.09.003
Maini R, Collison DJ, Maidment JM, Davies PD, Wormstone IM (2002) Pterygial derived fibroblasts express functionally active histamine and epidermal growth factor receptors. Exp Eye Res 74:237–244. https://doi.org/10.1006/exer.2001.1116
Cai Y, Zhou T, Chen J, Cai X, Fu Y (2023) Uncovering the role of transient receptor potential channels in pterygium: a machine learning approach. Inflamm Res 72:589–602. https://doi.org/10.1007/s00011-023-01693-4
Kaneko Y, Szallasi A (2014) Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol 171:2474–2507. https://doi.org/10.1111/bph.12414
Touyz RM (2008) Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol 294:H1103-1118. https://doi.org/10.1152/ajpheart.00903.2007
Alptekin M, Eroglu S, Tutar E, Sencan S, Geyik MA, Ulasli M, Demiryurek AT, Camci C (2015) Gene expressions of TRP channels in glioblastoma multiforme and relation with survival. Tumour Biol J Int Soc Oncodev Biol Med 36:9209–9213. https://doi.org/10.1007/s13277-015-3577-x
Liu T, Fang Z, Wang G, Shi M, Wang X, Jiang K, Yang Z, Cao R, Tao H, Wang X, Zhou J (2016) Anti-tumor activity of the TRPM8 inhibitor BCTC in prostate cancer DU145 cells. Oncol Lett 11:182–188. https://doi.org/10.3892/ol.2015.3854
Nilius B, Flockerzi V (2014) Mammalian transient receptor potential (TRP) cation channels. Preface Handb Exp Pharmacol 223:v–vi
Dehkordi O, Rose JE, Fatemi M, Allard JS, Balan KV, Young JK, Fatima S, Millis RM, Jayam-Trouth A (2012) Neuronal expression of bitter taste receptors and downstream signaling molecules in the rat brainstem. Brain Res 1475:1–10. https://doi.org/10.1016/j.brainres.2012.07.038
Okumus S, Demiryürek S, Gürler B, Coskun E, Bozgeyik I, Oztuzcu S, Kaydu E, Celik O, Erbagcı I, Demiryürek AT (2013) Association transient receptor potential melastatin channel gene polymorphism with primary open angle glaucoma. Mol Vis 19:1852–1858
Lam DS, Wong AK, Fan DS, Chew S, Kwok PS, Tso MO (1998) Intraoperative mitomycin C to prevent recurrence of pterygium after excision: a 30-month follow-up study. Ophthalmology 105:901–904; discussion 904–905. https://doi.org/10.1016/s0161-6420(98)95034-5
Dorovkov MV, Ryazanov AG (2004) Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem 279:50643–50646. https://doi.org/10.1074/jbc.C400441200
Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, Lehen’kyi V, Slomianny C, Beck B, Mariot P, Bonnal JL, Mauroy B, Shuba Y, Capiod T, Skryma R, Prevarskaya N (2006) Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Can Res 66:2038–2047. https://doi.org/10.1158/0008-5472.Can-05-0376
Faouzi M, Penner R (2014) TRPM2. Handb Exp Pharmacol 222:403–426. https://doi.org/10.1007/978-3-642-54215-2_16
Perraud AL, Knowles HM, Schmitz C (2004) Novel aspects of signaling and ion-homeostasis regulation in immunocytes. The TRPM ion channels and their potential role in modulating the immune response. Mol Immunol 41:657–673. https://doi.org/10.1016/j.molimm.2004.04.013
Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE (2009) TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal 2:ra21. https://doi.org/10.1126/scisignal.2000146
van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, Kamermans M (2009) Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet 85:730–736. https://doi.org/10.1016/j.ajhg.2009.10.012
İlhan HD, Ünal B, Ayaz Y, Erin N (2022) Changes in TRPV1 expression as well as substance P and vasoactive intestinal peptide levels are associated with recurrence of pterygium. Int J Mol Sci 23:15692
Acknowledgements
Not applicable.
Funding
This study was supported by Gaziantep University Scientific Research Projects Unit with project number TF.15.19.
Author information
Authors and Affiliations
Contributions
YT and SO conducted study and performed experiments and statistical analysis. YT, RG, SO and IE, wrote the paper. All authors read and edited final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
Approval for the study was granted by the Gaziantep University Clinical Research Ethics Committee (Protocol number: 2015/49).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tuylu, Y., Okumus, S., Gul, R. et al. High-throughput screening of transient receptor potential (TRP) channels in pterygium. Int Ophthalmol 44, 63 (2024). https://doi.org/10.1007/s10792-024-02938-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-02938-9