Abstract
Purpose
To evaluate the feasibility and effects of the intrastromal implantation of chemically modified corneal stroma obtained from chicken into the corneas of rabbits for corneal thickening.
Methods
Chicken corneas were cut, debrided, treated with cross-linking and implanted in an intrastromal pouch created in the cornea of 10 white New Zealand rabbits with femtosecond laser. Slit-lamp biomicroscopy and optical coherence tomography were performed immediately, 7, 30 and 90 days postoperatively. Corneas were removed at 90 days and cut in two halves. One half was sent to histological analysis for the presence of necrosis, polymorphonuclear inflammatory cells, blood vessels and fibrosis, while the other half was evaluated with transmission electron microscopy to verify tissue organization and the presence of keratocytes and inflammatory cells. Corneal thicknesses were comparatively analyzed over time with Wilcoxon test (p ≤ 0.05).
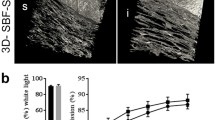
Results
The chicken grafts were incorporated into the cornea of all animals over time. Mean rabbit cornea thickness increased from 338 µm preoperatively to 538 µm (p < 0.0077) at 90 days, while mean chicken graft thickness decreased from 350 to 215 µm (p < 0.0077). No clear signs of rejection attributable to the xenograft were observed in any of the implanted eyes. However, some macroscopic and histological events were observed in some of the eyes, probably due to procedural issues during implantation.
Conclusion
The intrastromal implantation of chicken grafts was shown to be feasible and predictable to thicken the recipient rabbit cornea without apparent rejection. However, before being considered in humans, further meticulous clinical trials are required to establish the clinical utility, safety and efficacy of xenografts for the treatment of patients with advanced keratoconus.





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Rabinowitz YS (1998) Keratoconus. Surv Ophthalmol 42:297–319. https://doi.org/10.1016/s0039-6257(97)00119-7
Hamdi IM (2011) Preliminary results of intrastromal corneal ring segments implantation to treat moderate to severe keratoconus. J Cataract Refract Surg 37:1125–1132. https://doi.org/10.1016/j.jcrs.2010.12.048
Ashwin PT, McDonnell PJ (2010) Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol 94:965–970. https://doi.org/10.1136/bjo.2009.164228
Jhanji V, Sharma N, Vajpayee RB (2010) Management of keratoconus: current scenario. Br J Ophthalmol 95:1044–1050. https://doi.org/10.1136/bjo.2010.185868
Tan DT, Dart JK, Holland EJ, Kinoshita S (2012) Corneal transplantation. Lancet 379:1749–1761. https://doi.org/10.1016/S0140-6736(12)60437-1
Kelly TL, Williams KA, Coster DJ (2011) Australian corneal graft registry. Corneal transplant for keratoconus: a registry study. Arch Ophthalmol 129:691–697. https://doi.org/10.1001/archophthalmol.2011.7
Kremer I, Eagle RC, Rapuano CJ, Laibson PR (1995) Histologic evidence of recurrent keratoconus seven years after keratoplasty. Am J Ophthalmol 119:511–512. https://doi.org/10.1016/s0002-9394(14)71239-5
Tuft SJ, Buckley RJ (1995) Iris ischaemia following penetrating keratoplasty for keratoconus (Urrets-Zavalia syndrome). Cornea 14(6):618–622
Liu H, Zhu W, Jiang AC, Sprecher AJ, Zhou X (2012) Femtosecond laser lenticule transplant in rabbit cornea: experimental study. J Refract Surg 28:907–911. https://doi.org/10.3928/1081597X-20121115-05
Liu R, Zhao J, Xu Y, Li M, Niu L, Liu H, Sun L, Chu R, Zhou X (2015) Femtosecond laser-assisted corneal small incision allogeneic intrastromal lenticule implantation in monkeys: a pilot study. Invest Ophthalmol Vis Sci 56:3715–3720. https://doi.org/10.1167/iovs.14-15296
Zhao J, Shen Y, Tian M, Sun L, Zhao Y, Zhang X, Zhou X (2017) Corneal lenticule allotransplant after femtosecond laser small incision lenticule extraction in rabbits. Cornea 36(2):222–228. https://doi.org/10.1097/ICO.0000000000001076
Sekundo W, Kunert K, Russmann C, Gille A, Bissmann W, Stobrawa G, Sticker M, Bischoff M, Blum M (2008) First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six-month results. J Cataract Refract Surg 34:1513–1520. https://doi.org/10.1016/j.jcrs.2008.05.033
van Dijk K, Liarakos VS, Parker J, Ham L, Lie JT, Groeneveld-van Beek EA, Melles GR (2015) Bowman layer transplant to reduce and stabilize progressive, advanced keratoconus. Ophthalmology 122:909–917. https://doi.org/10.1016/j.ophtha.2014.12.005
Li M, Li M, Sun L, Han T, Ding L, Xiang J, Zhou X (2018) In vivo confocal microscopic investigation of the cornea after autologous implantation of lenticules obtained through small incision lenticule extraction for treatment of hyperopia. Clin Exp Optom 101:38–45. https://doi.org/10.1111/cxo.12595
Almodin EM, Ferrara P, Camin FMA, Colallilo JMA (2018) Femtosecond laser–assisted intrastromal corneal lenticule implantation for treatment of advanced keratoconus in a child’s eye. J Cataract Refract Surg 6:25–29. https://doi.org/10.1016/j.jcro.2018.01.004
Pascolini D, Mariotti SP (2010) Global estimates of visual impairment. Br J Ophthalmol 96:614–618. https://doi.org/10.1136/bjophthalmol-2011-300539
Alió Del Barrio JL, El Zarif M, de Miguel MP, Azaar A, Makdissy N, Harb W, El Achkar I, Arnalich-Montiel F, Alió JL (2017) Cellular therapy with human autologous adipose-derived adult stem cells for advanced keratoconus. Cornea 36:952–960. https://doi.org/10.1097/ICO.0000000000001228
Gonzalez-Andrades M, Sharifi R, Islam MM, Divoux T, Haist M, Paschalis EI, Gelfand L, Mamodaly S, Di Cecilia L, Cruzat A, Ulm FJ, Chodosh J, Delori F, Dohlman CH (2018) Improving the practicality and safety of artificial corneas: pre-assembly and gamma-rays sterilization of the Boston Keratoprosthesis. Ocul Surf 16:322–330. https://doi.org/10.1016/j.jtos.2018.04.002
Islam MM, Buznyk O, Reddy JC, Pasyechnikova N, Alarcon EI, Hayes S, Lewis P, Fagerholm P, He C, Iakymenko S, Liu W, Meek KM, Sangwan VS, Griffith M (2018) Biomaterials-enabled cornea regeneration in patients at high risk for rejection of donor tissue transplantation. NPJ Regen Me 3:2. https://doi.org/10.1038/s41536-017-0038-8
Isaacson A, Swioklo S, Connon CJ (2018) 3D bioprinting of a corneal stroma equivalent. Exp Eye Res 173:188–193. https://doi.org/10.1016/j.exer.2018.05.010
Zhang B, Xue Q, Li J, Ma L, Yao Y, Ye H, Cui Z, Yang H (2019) 3D bioprinting for artificial cornea: Challenges and perspectives. Med Eng Phys 71:68–78. https://doi.org/10.1016/j.medengphy.2019.05.002
Jin H, Liu L, Ding H, He M, Zhang C, Zhong X (2018) Small incision femtosecond laser-assisted x-ray-irradiated corneal intrastromal xenotransplantation in rhesus monkeys: a preliminary study. Curr Mol Med 18:612–621. https://doi.org/10.2174/1566524019666190129123935
Zheng X, Zhang D, Li S, Zhang J, Zheng J, Du L, Gao J (2018) An experimental study of femto-laser in assisting xenograft acellular cornea matrix lens transplantation. Med Sci Monit 27(24):5208–5215. https://doi.org/10.12659/MSM.909294
Schenke-Layland K et al (2003) Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol 143:201–208. https://doi.org/10.1016/j.jsb.2003.08.002
Fowler WC, Chang DH, Roberts BC, Zarovnaya EL, Proia AD (2004) A new paradigm for corneal wound healing research: the white leghorn chicken (Gallus gallus domesticus). Curr Eye Res 28:241–250. https://doi.org/10.1076/ceyr.28.4.241.27837
Almodin CG, Minguetti-Camara VC, Meister H, Ferreira JO, Franco RL, Cavalcante AA, Radaelli MR, Bahls AS, Moron AF, Murta CG (2004) Recovery of fertility after grafting of cryopreserved germinative tissue in female rabbits following radiotherapy. Hum Reprod 19:1287–1293. https://doi.org/10.1093/humrep/deh246
Cerialle PMA, Almodin CG, Radaelli MRM, Minguetti-Câmara VC, Souza MC, Oliveira CAM, Gonçalves AJ (2017) Viability of homologous and heterologous subcutaneous transplant of fresh germ cells in rabbits. JBRA Assist Reprod 21:73–78. https://doi.org/10.5935/1518-0557.20170019
Bromley JG, Randleman JB (2010) Treatment strategies for corneal ectasia. Curr Opin Ophthalmol 21:255–258. https://doi.org/10.1097/ICU.0b013e32833a8bfe
Akhtar S, Bron AJ, Salvi SM, Hawksworth NR, Tuft SJ, Meek KM (2008) Ultrastructural analysis of collagen fibrils and proteoglycans in keratoconus. Acta Ophthalmol 86:764–772. https://doi.org/10.1111/j.1755-3768.2007.01142.x
Suwan-Apichon O, Reyes JM, Griffin NB, Barker J, Gore P, Chuck RS (2006) Microkeratome versus femtosecond laser predissection of corneal grafts for anterior and posterior lamellar keratoplasty. Cornea 25:966–968. https://doi.org/10.1097/01.ico.0000226360.34301.29
Mian SI, Shtein RM (2007) Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol 18:295–299. https://doi.org/10.1097/ICU.0b013e3281a4776c
Shousha MA, Yoo SH, Kymionis GD, Ide T, Feuer W, Karp CL, O'Brien TP, Culbertson WW, Alfonso E (2011) Long-term results of femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology 118:315–323. https://doi.org/10.1016/j.ophtha.2010.06.037
del Barrio JLA, Chiesa M, Garagorri N, Garcia-Urquia N, Fernandez-Delgado J, Bataille L, Rodriguez A, Arnalich-Montiel F, Zarnowski T, de Toledo JPÁ, Alio JL, De Miguel MP (2015) Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res 132:91–100. https://doi.org/10.1016/j.exer.2015.01.020
Cissell DD, Hu JC, Griffiths LG, Athanasiou KA (2014) Antigen removal for the production of biomechanically functional, xenogeneic tissue grafts. J Biomech 47:1987–1996. https://doi.org/10.1016/j.jbiomech.2013.10.041
Sharifi R, Yang Y, Adibnia Y, Dohlman CH, Chodosh J, Gonzalez-Andrades M (2019) Finding an optimal corneal xenograft using comparative analysis of corneal matrix proteins across species. Sci Rep 9:19876. https://doi.org/10.1038/s41598-018-38342-4
Li A, Zhang Y, Liu Y, Pan Z (2017) Corneal xenotransplantation from pig to rhesus monkey: no signs of transmission of endogenous porcine retroviruses. Transplant Proc 49:2209–2214. https://doi.org/10.1016/j.transproceed.2017.07.018
Badylak SF (2014) Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng 42:1517–1527. https://doi.org/10.1007/s10439-013-0963-7
Isidan A, Liu S, Li P, Lashmet M, Smith LJ, Hara H, Cooper DKC, Ekser B (2019) Decellularization methods for developing porcine corneal xenografts and future perspectives. Xenotransplantation 28:e12564. https://doi.org/10.1111/xen.12564
Dana MR, Qian Y, Hamrah P (2000) Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 19:625–643. https://doi.org/10.1097/00003226-200009000-00008
Maguire MG, Stark WJ, Gottsch JD, Stulting RD, Sugar A, Fink NE, Schwartz A (1994) Risk factors for corneal graft failure and rejection in the collaborative corneal transplant studies; Collaborative Corneal Transplant Studies Research Group. Ophthalmology 101:1536–1547. https://doi.org/10.1016/s0161-6420(94)31138-9
Qazi Y, Hamrah P (2013) Corneal allograft rejection: immunopathogenesis to therapeutics. J Clin Cell Immunol. https://doi.org/10.4172/2155-9899.S9-006
Lindstrom RL, Macrae SM, Pepose JS, Hoopes PC Sr (2013) Corneal inlays for presbyopia correction. Curr Opin Ophthalmol 24:281–287. https://doi.org/10.1097/ICU.0b013e328362293e
Böhm M, Shajari M, Remy M, Kohnen T (2019) Corneal densitometry after accelerated corneal collagen cross-linking in progressive keratoconus. Int Ophthalmol 39:765–775. https://doi.org/10.1007/s10792-018-0876-4
Lopes B, Ramos I, Ambrósio R (2014) Corneal densitometry in keratoconus. Cornea 33:1282–1286. https://doi.org/10.1097/ICO.0000000000000266
Acknowledgements
The authors would like to express their gratitude to Mr. Antonio Carlos Correa for reviewing the English version of the paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FA, JA and EA, and NF under the supervision of PF and AG. The first draft of the manuscript was written by FA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from Animal Research Ethics Committee of Ingá Animal Medical School (UNINGA), Maringá, Brazil (No. PM 06/2017). The procedures used in this study adhere to the criteria recommended by the Brazilian College of Animal Experimentation (COBEA) and the ARRIVE Guidelines for Reporting Animal Research.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almodin, F.M., Almodin, J.M., Almodin, E.M. et al. Intrastromal implantation of chicken corneal grafts into the cornea of rabbits for corneal thickening: an experimental study. Int Ophthalmol 41, 243–255 (2021). https://doi.org/10.1007/s10792-020-01573-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01573-4