Abstract
The severe acute respiratory syndrome coronavirus 2 has been a shocking disaster for healthcare systems worldwide since December 2019. This virus can affect all systems of the body and its symptoms vary from a simple upper respiratory infection to fatal complications including end-organ damage. On the other hand, the normal immune system plays a pivotal role in the recovery of infectious diseases such as COVID-19. However, occasionally, exaggerated immune system inflammation and an excessive synthesis of cytokines, known as a "cytokine storm," can deteriorate the patient's clinical condition. Secondary bacterial co-infection is another problem in COVID-19 which affects the prognosis of patients. Although there are a few studies about this complication, they suggest not using antibiotics commonly, especially broad-spectrum ones. During this pandemic, various approaches and therapeutics were introduced for treating COVID-19 patients. However, available treatments are not helpful enough, especially for complicated cases. Hence, in this era, cell therapy and regenerative medicine will create new opportunities. Therefore, the therapeutic benefits of mesenchymal stem cells, especially their antimicrobial activity, will help us understand how to treat COVID-19. Herein, mesenchymal stem cells may stop the immune system from becoming overactive in COVID-19 patients. On the other side, the stem cells' capacity for repair could encourage natural healing processes.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first was identified in China in 2019, which spreads all over the world and causes the coronavirus pandemic. Coronavirus disease- 2019 (COVID-19) can have a wide range of symptoms ranging from asymptomatic cases to patients with severe forms of COVID-19. Accordingly, while some cases are asymptomatic and some patients develop a viral upper respiratory infection, other individuals face life-threatening symptoms such pneumonia, respiratory failure, shock, and multi-organ dysfunction (Arjmand et al. 2020a; Azodi et al. 2020; Batu and Özen 2020; Aghili et al. 2021; Azodi et al. 2021). Additionally, cytokine storm or cytokine release syndrome (CRS), overproduction of cytokines, and bacterial co-infection or secondary bacterial infection are examples of lethal complications of COVID-19 (Feng et al. 2020; Rana 2020, Moezzi et al. 2021). Herein, while COVID-19 is mostly transmitted through the lungs, it has been demonstrated to also have gastrointestinal effects, with diarrhea being reported in roughly one-third of patients. Accordingly, bacterial transmission via the gastrointestinal tract has the potential to exacerbate severe COVID-19 infection and hyper inflammation (Wang et al. 2020; Sahu et al. 2021; Ghazanfar et al. 2022). Indeed, hyper inflammation and cytokine storm can worsen the patients’ conditions, especially in patients with prior immune system problems. In this respect, the pieces of literature have been shown that these severe complications are associated with immune-related and rheumatology diseases including rheumatoid arthritis (RA). Nevertheless, during this pandemic, managing COVID-19 and severe concurrent issues in addition to their past immunosuppressive medications becomes a challenge (Rana 2020; Song et al. 2020). Actually, secondary bacterial infections have been seen not only in COVID-19 but also in other viral respiratory infections such as influenza. Though the overall rate of this complication is low, its proportion rate among the critical patients is slightly high. Accordingly, rapid diagnosis and on-time treatment is a vital to point in these cases. Additionally, similar to other infections, contacts, environmental exposures, airborne and droplets from other patients, as well as healthcare professionals, could spread opportunistic organisms to highly sensitive patients and result in secondary infection in these patients. Thus, environmental exposures during the home or health-care center isolation, hospital admission, and even convalescence time are highly affect the secondary infections rate (Hamel et al. 2010; Rice et al. 2012, Langford et al. 2020, Rawson et al. 2020). Herein, although routine therapeutics including corticosteroids and antibiotics are used to manage these problems, they cannot cure these patients (Fu et al. 2020; Vaillancourt and Jorth 2020). Accordingly, new treatments and techniques such as regenerative medicine (RM) and cell therapy are required to improve the management of these patients (Saeedi et al. 2019; Arjmand et al. 2021). In light of the information provided, this review will explore the immunopathology and pathophysiological mechanisms of COVD-19, comorbidities such as secondary and co-bacterial infection, and potential novel therapeutic methods.
COVID-19: immunopathology and pathophysiological mechanisms
Actually, immune system activates against the SARS-CoV-2 and its antiviral responses limit viral infection in human body. However, SARS-CoV-2 by various mechanisms can damage the normal immune response which causes the critical morbidities. Accordingly, it will be possible to develop novel medicines, improved management techniques, and efficient vaccinations as a result of new insights into the immunopathology and pathophysiological mechanisms of this disease. Subsequently, the authors are summarized the opposing and protective functions of immunity in COVID-19(Alrubayyi 2020).
Innate and adaptive immune responses to SARS-CoV-2 infection
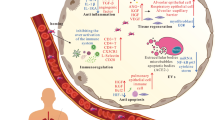
SARS-CoV-2 is a new strain of the Coronaviridae family of viruses that causes the various types of COVID-19 from asymptomatic to severe and lethal forms. Accordingly, the immune system through different mechanisms combat SARS-CoV-2 infection. Some references has shown that in an early phase of infection, SARS-CoV-2 multiplies rapidly in the body by suppressing the innate immune system. However, in late phases body’s immune system striking responses can lead to severe complications including cytokine storm and acute respiratory distress syndrome (ARDS) (Chowdhury et al. 2020; García 2020; Huang et al. 2020; Soleimanian and Yaghobi 2020). Angiotensin-converting enzyme 2 (ACE2) can be found in the membrane of different cells of human organs. ACE 2 as a part of Renin Angiotensin Aldosterone System (RAAS) has an important role in controlling human’s blood pressure. Indeed, ACE 2 activates a cascade of hormonal reactions in RAAS by converting angiotensin I to angiotensin II. On the other hand, SARS-CoV-2 enter host cells through ACE 2 and spread to various organs by free infected cells (Horiuchi et al. 1999; Wrapp et al. 2020). Additionally, studies have shown that SARS-CoV-2 downregulates the ACE 2 receptor on cell membranes which leads to imbalance of various parts of RAAS and finally organ damage (Kuba et al. 2005). During SARS-CoV-2 infection, innate immune response activation leads to antiviral interferons and different chemokines production and attracts more innate cells including polymorphonuclear leukocytes, monocytes, natural killer (NK) cells, and dendritic cells (DCs) to the infective sites. Additionally, these mechanisms help to recruit more lymphocytes and provide potent help to antiviral activities of the innate response (Dandekar and Perlman 2005; Chen and Subbarao 2007). Also, adaptive response assists the innate system to eliminate the virus through neutralizing antibody production by B cells and the cytolytic effect of CD8 T cells. Additionally, CD4 T cells contribute to an adaptive response by various mechanisms including other lymphoid cell recruitment, helping B cells to provide high-affinity antibodies, CD8 T cell priming and etc. (Fig. 1; Sant and McMichael 2012; García 2020). As well, evidence has shown the immune response is considerably different in various clinical manifestations of COVID-19. For example, although in mild forms of COVID-19 innate NK-cells and transient short-lived Ig A, M, G antibodies play a pivotal role against the viral infection, in severe forms large amounts of monocytes and long-lived Ig A, G antibodies are important (Carsetti et al. 2020). Interestingly, NK cells as a frontline in the immune response against COVID-19 are a bridge between innate and adaptive immune responses. In this regard, a subset of NK cells called NKG2C + cells carry out their cytotoxic effect and pro-inflammatory effector molecules released by enhancing the activity of the CD94/NKG2C receptor and its HLA-E ligand (Vietzen et al. 2020). Unfortunately, sometimes NK-cell cytolytic effect is damaged by virus interaction and cytokine storm effect. Hence, researchers suggest cytolytic NK-cell transferring as a potential therapeutic method in COVID-19 (Ghasemzadeh et al. 2021). Collectively, a better understanding of the immune response against SARS-Cov-2 will promote the treatments and vaccines.
Innate and adaptive immune response against SARS-CoV-2. SARS-CoV-2 enters host cells via ACE 2 receptors. Then virus begins to replicates in the living cell which induces the inflammation. Furthermore, innate immune cells, NK cells, INF I, and various interleukins together help to induce humoral and cellular responses (León-Rodríguez et al. 2020)
Hyper inflammation and cytokine storm; signaling pathways
Generally, cellular signaling networks are required for replication, translation, nuclear membrane transport, capsid assembly, and dispersion, as well as the reactivation of virus latency to produce infectious virus (Chi and Liu 2013). On the other hand, the immune system is equipped with a sophisticated mechanism that can respond to a variety of infections (Klimpel 1996; Chaplin 2010). Herein, the immune system’s inflammatory pathways will be activated to produce a normal antiviral response (Fig. 2). Excessive inflammatory response to the SARS-CoV-2 virus is followed by the releasing a large number of pro-inflammatory cytokines. The mention life-threatening condition is known as CRS. During days 8 to 12 of the infection, some people with COVID-19 pneumonia suffer from CRS. According to clinical definitions, they require oxygen therapy and have a fever, exhaustion, anorexia, headaches, diarrhea, dyspnea, coughing, and cyanosis (Marc et al. 2022). Indeed. The CRS has been recognized as a significant cause of death in COVID-19 individuals (Ragab et al. 2020; Sinha et al. 2020; Yang et al. 2021). In this respect, according to some evidence, when cytokine levels in the plasma of COVID-19 reported cases were compared to healthy individuals, higher levels of cytokines such as interleukin (IL)-6, IL-7, IL-10, granulocyte–macrophage colony-stimulating factor (GM-CSF), interferon(IFN-), tumor necrosis factor(TNF-), and etc. were found (Han et al. 2020; Arjmand et al. 2021). Moreover, there is a theory that microorganisms that promote shedding of membrane receptors and boost endogenous shedding mechanisms can play a role in the development of CRS (Földvári-Nagy et al. 2021).
Signaling Pathways and COVID-19. TMPRSS2 is one of the type-II transmembrane serine proteases (TTSPs) that cleaves the viral spike protein to reveal the fusion peptide for cell entrance. These TTSPs play a crucial role in the virus lifecycle. The binding of the spike glycoprotein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to angiotensin-converting enzyme 2 (ACE2) which is shedding by ADAM17 (A disintegrin and metalloprotease 17) can cause a dysregulation of the renin–angiotensin system (RAS), favoring the ACE–angiotensin II (Ang II)–angiotensin II type I transmitter (AT1R) axis. The ACE2 enzyme transforms Ang II into a heptapeptide (Ang 1–7) 7(When it binds to a specific receptor in the body, it has positive effects such as vasodilation, anti-thrombotic, anti-fibrotic, and anti-inflammatory) and Ang I into a nonapeptide (Ang 1–9) as a master regulator of the RAS signaling pathway. AT1R, G protein-coupled receptor (GPCR) family member, regulates the harmful effects of Ang II by activating several downstream signaling pathways, for instance MAP kinases (p38MAPK), receptor tyrosine kinases (Epidermal growth factor receptor (EGFR)), and non-receptor tyrosine kinases (Janus tyrosine kinase (JAK)—signal transducers and activators of transcription (STAT) (JAK/STAT)).When ACE2 is depleted, Ang II is overproduced, and its attachment to AT1R activates the ADAM17 protease. ADAM17 can cleave membrane-anchored proteins and immunological cytokines e.g.,interlukin-6 (IL-6), tumor necrosis factor alpha (TNF- α), and EGFR ligands, to initiate pro-inflammatory pathways. In addition, ADAM17 cleaves the Notch-ligand complex, and the -secretase complex cleaves the Notch intracellular domain, resulting in Notch release and transport to the nucleus, as well as transcriptional activation of Notch target genes such as inflammatory cytokines and furin. In the lungs, Des-arg9 bradykinin (DABK) is a biological substrate of ACE2, and ACE2 deficiency resulted in DABK activation of the bradykinin receptor (B1R) and the release of pro-inflammatory chemokines. Furthermore, B1R activation can result in AT1R overexpression, while ADAM17 stimulation can result in EGFR transactivation. The expression of B1R can be considerably increased by Ang II stimulation, implying probable cross-talk between AT1R and B1R in SARS-CoV-2 infection (de Queiroz et al. 2020; Furuhashi et al. 2020; Zipeto et al. 2020, Farahani et al. 2022). BioRender was used to create this image
JAK/STAT signaling cascade
According to investigations, the Janus tyrosine kinase (JAK)—signal transducers and activators of transcription (STAT) signaling pathway could be involved in the downstream effects of ACE2 hyperactivation (Hu et al. 2021; Luo et al. 2021). On the other hand, the JAK-STAT pathway has a role in immune system coordination, especially cytokine receptors, and can regulate T helper cell polarization. JAKs (e.g., Jak1, Jak2, Jak3, and tyrosine kinase 2 (Tyk2)) bind to the cytoplasmic domains of type I and II cytokine receptors (Seif et al. 2017; Luo et al. 2021). When cytokine receptors phosphorylate JAKs, subsequently STATs (STAT1-6) are phosphorylated and trafficked into the nucleus to translate inflammatory mediators. Suppressors of cytokine signaling (SOCS), protein inhibitors of activated STATs (PIAS), and protein tyrosine phosphatases (PTPs) are among the regulator proteins that control the beginning, length, and termination of mentioned signaling cascades(Xu and Qu 2008; Krämer and Heinzel 2010; Seif et al. 2017). IL2-7 and IL-12, GM-CSF, growth hormone (GH), epidermal growth factor (EGF), platelet-derived growth factors (PDGF), and IFNs are only a few of the cytokines and growth factors that send signals through the JAK-STAT pathway(Bousoik and Montazeri Aliabadi 2018; Hu et al. 2021; Yin et al. 2021). Accordingly, there is some evidence that inhibiting the JAK -STAT pathway can be a therapeutic option for COVID-19 treatment. In this context, multiple clinical trials are under planned to investigate the possible use of JAK -STAT pathway inhibitors in treating patients with COVID-19-associated CRS (Alijotas-Reig et al. 2020; Satarker et al. 2021).
NF-κB signaling cascade
The nuclear factor kappa-light-chain enhancer of activated B cells (NF-kappaB) (NF-κB) family of transcription factors is involved in immunity, inflammation, and cellular development. The Rel-like domain-containing proteins p65/RelA, RelB, c-Rel, NF-κB 1, and NF-κB 2 belong to the NF-κB family (Oeckinghaus and Ghosh 2009; Gelmann et al. 2013). NF-κB is an upstream regulator of IL-1, IL-2, IL-6, IL-12, TNF-, LT-, GM-CSF, and other chemokines production in different cells e.g., macrophages in the central nervous, cardiovascular, and gastrointestinal system, lungs, liver, and kidney (Hariharan and Hakeem 2021). AS the other crucial point, the NF-κB has long been recognized as a disease-causing factor and it is involved in inflammation. Herein, during SARS-CoV-2 prevalence, virus proteins triggered enhanced NF-κB activation, resulting in high disease severity and death. Therefore, NF-κB pathway could be a target for therapeutic interventions (Li et al. 2021).
NLRP3 signaling cascade
Nucleotide-binding oligomerization domain-like receptors (NLRs) are intracellular pattern recognition proteins. They can recognize pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), or other signals in the cytosol (Franchi et al. 2006). Since this is necessary for immune responses to invading pathogens, inflammasome activation of the NLRs (containing pyrin domain 3 (NLRP3)) stimulates the release of cytokines as a response to viral infection. Herein, the formation of a cytokine storm in severe COVID-19 individuals reveals that the NLRP3 inflammasome is involved in COVID-19 (Batiha et al. 2021; Zhao et al. 2021). Generally, viroporins, ion flux, and other complicated molecular processes activate the NLRP3 inflammasome in response to SARS-CoV-2 infection. Moreover, NLRP3 inflammation is linked to the onset of respiratory, cardiovascular, and neurological symptoms in COVID-19. Accordingly, inhibitors that target the NLRP3 inflammasome and its downstream pathways are among the novel attractive therapeutic intervention for COVID-19 (Maes et al. 2021; Zhao et al. 2021).
MAP kinase signaling cascade
The mitogen-activated protein kinase (MAPK) as a family of highly conserved serine-threonine protein kinases (e.g., extracellular signal-regulated kinase (ERK), p38, and c-Jun NH (2)-terminal kinase (JNK)) connects cell surface receptors to the transcription machine and converts extracellular inputs, such as viral infections, to the nucleus of the cell, and then to a variety of outputs that could help the host responding to viral infections(Roux and Blenis 2004; Cuenda 2019). Moreover, in virus-infected cells, the MAPK pathway is also involved in controlling the immune reaction and apoptosis. On the other hand, MAPK signaling can act as a positive or negative modulator of viral replication. In this context, based on some investigations, the MAPK signaling pathway can be considered a plausible therapeutic target because of its involvement in the viral infection (Kumar et al. 2018; Yue and López 2020).
Notch signaling cascade
Notch signaling, a major regulator of the cardiovascular system, has been linked to a range of viral infection-related biological processes. Indeed, Notch pathway regulates a variety of proliferative and differentiation processes in cells, so it's no wonder that viruses that rely on the cell cycle machinery find it appealing (Rizzo et al. 2020; Breikaa and Lilly 2021; Trivedi et al. 2021). FURIN is a Notch activator that belongs to the protein convertases family. It has been discovered that a variety of viruses, including measles, yellow fever, ebola, and avian influenza, employ its enzymatic activity to boost their pathogenicity and spread. In the case of COVID-19, SARS-CoV-2 S protein has two purposes: it binds the receptor and mediates the viral particle's integration into the cell membrane. FURIN as a protease, cleaves Protein S, exposing the fusion sequences and allowing them to enter the cell (Braun and Sauter 2019, Racaniello et al. 2020; Dayer 2021). Generally, researchers believe Notch could be utilized to combat heart and lung disease induced directly by SARS-CoV-2 infection and the cytokine storm that occurs in response to the virus (Breikaa and Lilly 2021).
Bacterial infections in COVID-19 patients
Several cell types, including lung epithelial cells and enterocytes (in the ileum and colon), can express the ACE2 receptor. Enterocytes serve as columnar cells that make up the majority of the gastrointestinal intestine's epithelium. On the other hand, bacteria can transmit via the gastrointestinal tract; therefore, they have the potential to exacerbate severe COVID-19 infection by occurring bacterial infection. Serratia marcescens, Staphylococcus aureus, Pseudomonas aeruginosa, Listeria monocytogenes and etc. are some of the bacteria that typically cause bacterial infections during viral pneumonia (Fattorini et al. 2020; Földvári-Nagy et al. 2021). Long-standing research has shown that bacterial infections can exacerbate the consequences of viral respiratory symptoms. In this respect, co-infections, secondary infections, and/or superinfections may play different roles in patients with COVID-19 infection, although it is yet unknown exactly how they do so. Generally, studies indicated that, the major number of co-infected individuals who died were infected with gram-negative bacteria. They can either directly promote membrane receptor shedding or boost natural shedding mechanisms (Mahmoudi 2020). Bacterial co-infections, due to their effects on morbidity and mortality of COVID-19 patients, have become a hot topic during this pandemic. Indeed, the mechanisms and associations of this event are not clear, but impaired respiratory epithelial integrity, the decline in mucociliary clearance, and increasing bacterial colonization in the respiratory tract are some of the possible explanations (Chertow and Memoli 2013; Goncalves Mendes Neto et al. 2021). Furthermore, it is discovered that endotoxin, a component of gram-negative bacteria's cell walls which has been widely studied and identified as one of the principal causes of fatal shock during severe sepsis, is also one of the main causes of the cytokine storm (Fig. 3). However, extra studies are required to clarify the exact mechanisms and risk factors of bacterial infections.
Bacterial Infections in COVID-19 Patients. First, SARS-CoV-2 infects type 2 pneumocytes in the lungs. Then, causing pneumonia and (in progressive stage) acute respiratory distress syndrome (ARDS) as well as increasing vulnerability to bacterial infection by weakening the pulmonary immune response. In organs with high levels of angiotensin-converting enzyme 2 (ACE2), such as the gastrointestinal organs, the virus can enter the bloodstream and induce viremia. Pathogenic bacteria from the gut lumen can translocate into the circulation due to changes in the intestinal microenvironment (Sirivongrangson et al. 2020). BioRender was used to create this image
Risk factors for bacterial infections
With respect to the previous experiences in flu outbreaks, co-infections are one of major problems during such pandemics. The prevalence of secondary bacterial infections is not high, albeit they increase the mortality and morbidity rate of COVID-19 cases. Also, co-infections pose a challenge to the creation of a regimen with least side effects and antibacterial resistance and improving the prognosis of these patients. Accordingly, during COVID-19 pandemic we face with various co-infections especially with bacterial ones, but the number of patients with co-infections are less than previous pandemics (Fattorini et al. 2020). In this regard, studies have estimated the bacterial infection rate ranging from 5.9 to 8.1% among all and critically ill patients (Langford et al. 2020). On the other hand, the rate of co-infections are higher in severely ill patients and critically ill patients are more susceptible to secondary bacterial infections (Fattorini et al. 2020; Feldman and Anderson 2021; Pourajam et al. 2022). Hence, patient’s condition can be considered an important risk factor in co-infections occurrence. However, a body of literature with regard to less burden of co-infections in this pandemic suggests that applying prophylactic antibiotics in patients seems unreasonable. Also, super-infection by nosocomial antibiotic-resistant bacteria is an alarm for preventing from routine use of broad-spectrum antibiotics in these settings (Fattorini et al. 2020; Isaacs and Burmester 2020; Lansbury et al. 2020; Vaillancourt and Jorth 2020; Zhu et al. 2020; Farrell et al. 2021). Furthermore, some articles have found that co-infections inhibit the immune system which deteriorates the patients’ condition. As well as, special groups with weak immune systems and previous underlying lung problems including chronic obstructive pulmonary disease (COPD) have higher risk of experiencing co-infections. For example, when severe underlying conditions in elderly people are accompanied with prolonged hospitalization make these patients more prone to such co-infections (Fattorini et al. 2020; Sharifipour et al. 2020; Westblade et al. 2021). Additionally, such as other infections, contact is one of important ways of transmission of infections. For instance, direct contact with a person who is infected or colonized with opportunistic microorganisms, indirect contact with contaminated tools such as intubation instruments and dressings, droplets from infected patients during sneezing, coughing, suctioning and intubation process stand accused of causing secondary infection in hospitalized patients (Hamel et al. 2010; Esposito et al. 2020). Moreover, concerning to rate of ventilator-associated bacterial pneumonia (VAP), mechanical ventilation though to its supportive care in COVID-19 patients with ARDS it predisposes these patients to the development of VAP (Póvoa et al. 2020). Also, mentioned factors can cause secondary bacterial infections not only during the illness period but also in convalescence time. Accordingly, avoiding close contact and proper self-quarantine is recommended for suspected or diagnosed with COVID-19 patients. Shoukat and colleagues have studied the benefits of self-isolation during this pandemic. Their results have demonstrated that self-isolation could delay the outbreaks peaks, ICU admission, and burden on health-care facilities (Shoukat et al. 2020). As well as, it seems self-isolation not only prevent the spreading disease to other people, but also it reduces the possible transmission of other opportunistic infections. Since, COVID-19 affect various organs of body which make them susceptible to secondary infections during and after their disease period. Recently, routine immunosuppressive treatments unlike to their effectiveness in severe COVID-19 are introduced as an risk factor for secondary infections (Hocková et al. 2021). Hereupon, further studies are required to determine more possible risk factors to better control and prevention of co-infections.
The most common bacterial infections
Previously, the common possible mechanisms and risk factors of co-infections are discussed. Commonly, SARS-CoV-2 infects the respiratory and lung cells. Therefore, like other viruses it can cause viral and secondary bacterial pneumonia and other respiratory complications (Vaillancourt and Jorth 2020). Different factors make the patient prone to co-infection with different pathogens. In a study by Zhu et al., respiratory pathogens as co-infections in COVID-19 cases are described by RT-PCR. Their investigation has shown that bacterial infections are more common than viral and fungal ones. Overall, among various bacterial infections Staphylococcus aureus and Streptococcus pneumonia are more frequent than Klebsiella pneumoniae and Haemophilus influenza infections (Zhu et al. 2020; Chaudhry et al. 2021). In addition to typical pathogens, atypical bacteria including Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila can cause co-infections whit similar clinical and imaging manifestations. Since the patients with atypical bacterial co-infections are more susceptible to develop ARDS, require for mechanical ventilator, and prolonged hospital and ICU admission, on time recognizing and effective antibiotic regimen seems to be necessary in possible co-infection cases (Chaudhry et al. 2021). Additionally, the great number of antibiotic-resistant bacterial infections are another challenge during this pandemic that antibiotic stewardship may solve this problem to some extent. Oral complications are another issue in COVID-19 patients which can become a life-long or even a dangerous problem. According to epidemiological results, perioral pressure ulcers, followed by oral candidiasis are more common. Such problems are more prevalent in ICU-as patients due to some risk factors including long prone position and ventilation devices around and in their mouth and nose (Hocková et al. 2021; Orilisi et al. 2021). Hence, an accurate care with attention to possible secondary infections manifestations and multidisciplinary management of these patients are required to eliminate the possible risk factors and improve their prognosis.
Therapeutic interventions
During the recent pandemic, various therapeutic interventions with antiviral and immunomodulatory properties are recommended for COVID-19. Actually, the food and drug administration (FDA) fully approves only some of them, however, some others do not have FDA approval. Indeed, during this public health emergency different therapeutics are suggested to apply with considerations by health authorities. Anyway, more studies are required to shed light on this field (Sanders et al. 2020; Niknam et al. 2022).
Drugs
Since this outbreak, various protocols and treatments are announced to treat and manage the COVID-19 cases. Although they have assisted to treat the patients, they cannot cure all patients and improve all of their COVID-19 related complications. With regards to the association of inflammation with complications of SARS-CoV-2, immunomodulatory and immunosuppressive therapies are promising drugs. Accordingly, remdesivir, nucleotide prodrug of an adenosine analog, is approved by FDA for the treatment of COVID-19. This drug inhibits viral replication via binding to the viral RNA-dependent RNA polymerase. However, due to its adverse effects on the liver and kidney, monitoring of their function is required in patients (Williamson et al. 2020; Ghandour et al. 2021). Studies about the CRS have shown that among the overproduction of various interleukins, the rising level of IL-6 is more associated with the mortality rate. Hence, FDA-approved anti-IL-6 receptor monoclonal antibodies including tocilizumab (TCZ) and sarilumab (SAR) through halting IL-6 action may improve patients’ condition. In this regard, TCZ and SAR are examples of monoclonal therapies which block the signaling cascade of IL-6 (Giamarellos-Bourboulis et al. 2020; Hojyo et al. 2020). As well, corticosteroids with anti-inflammatory effects are widely used in severely ill patients, especially with acute respiratory distress syndrome (ARDS) (Rezk and Ibrahim 2013). In this regard, combination therapy of corticosteroids with other antiviral drugs including tocilizumab and remdesivir is recommended for severe patients. On the other hand, the goals of antimicrobial stewardship should be incorporated into COVID-19 patient care pathways. Herein, individuals with COVID-19 and a life—threatening co-bacterial respiratory infection should start a five-day antibiotic treatment until their signs, symptoms, and systemic inflammation improve. Excessive use of antibiotics increases the risk of the patient reverting to pre-antibiotic status and developing antibiotic resistance. In general, the majority of drug treatments for COVID-19 target the signaling pathways involved in these diseases (Table 1). Additionally, studies in RM and cell therapy have recommended some possible treatments. (Golchin et al. 2020). In addition to various therapeutics, vaccine as a primary prevention was a hot topic. At this time, different types of vaccines with various mechanisms are available globally. Finally, both novel therapeutics agents and vaccines are required to effectively manage patients and counteract the consequence of uncontrolled immune responses like cytokine storm.
Regenerative medicine therapeutics
RM involves the application of various FDA-approved therapeutics i.e., autologous or allogeneic stem cells, NK cells, extracellular vesicles (EVs), and tissue products, as well as different combinations of these, to effectively replace missing tissue, both structurally and functionally, or to aid tissue regeneration (Mao and Mooney 2015; Golchin and Farahany 2019; Goodarzi et al. 2019b; Arjmand et al. 2020b). Mesenchymal stem cells (MSCs) are currently one of the most studied therapeutic cellular products in RM (Larijani et al. 2014; Goodarzi et al. 2018; Arjmand et al. 2019). Previous studies have demonstrated that MSCs are effective in treating a range of diseases. For instance, MSCs have the ideal characteristics for cardiovascular repair and can protect the myocardium in the cardiovascular system by lowering inflammation, fostering myocardial cell differentiation around infarct areas and angiogenesis, increasing apoptosis resistance, and inhibiting fibrosis. On the other hand, MSCs are promising candidates for the therapy of a number of digestive system diseases due to their potent immunomodulatory and regenerative characteristics. Moreover, for people with end-stage liver disease, MCS therapy has been seen as a possible alternative treatment option. Also, MSCs are particularly relevant as stem cell therapeutics in the treatment of cancer due to their capacity for immunomodulation and tumor-homing. However, limited progress has been made in translational medicine due to ignorance of the contentious roles that MSC play in the interaction with malignancies. Specifically, recent evidence suggests that MSC may play a therapeutic function in lung disorders and infections (Huang 2015; White et al. 2016; Cruz and Rocco 2020; Shi et al. 2020; Lan et al. 2021; Hoang et al. 2022). Accordingly, transplantation of MSCs and MSC-derived EVs are the main RM-based auxiliary treatments for COVID-19 that have been accepted by physicians or in clinical trials. Moreover, infusion of convalescent plasma is another RM -based treatment for COVID-19 (Parhizkar Roudsari et al. 2020; Arjmand et al. 2021; Gilany et al. 2021).
Effects of mesenchymal stem cells
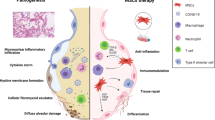
Within hours of starting an inflammatory response, toll-like receptors (TLRs) on innate effector cells recognize pathogen-produced compounds. TLR ligation may activate phagocytic cells as well as stromal cells (such as MSCs) resulting in an inflammatory response (Auletta et al. 2012; Rahmani-Kukia et al. 2020). Herein, MSCs polarize into two effective phenotypes (MSC1 or pro-inflammatory phenotype and MSC2 or immune-suppressive phenotype), each with its own immune modulatory actions and secretome. In this respect, many studies are concentrating on the use of MSCs in cell therapy. In other words, MSCs are currently being tested in clinical trials for the treatment of a wide range of incurable diseases and they show extremely promising results due to their rapid proliferation, immunomodulation effects, high differentiation capacity, and secretion of useful growth factors, ability to migrate into the site of damage, and free of ethical and social issues (Waterman et al. 2010; Rivera-Cruz et al. 2017; Goodarzi et al. 2019a; Gilany et al. 2021). They have the ability to self-renew and differentiate into a variety of mesenchymal lineages, including bones, cartilages, adipose tissues, and tendons. Furthermore, they can be isolated from a wide range of fetal and adult tissues (Larijani et al. 2015; Goodarzi et al. 2019a; Abedi et al. 2020; Aghayan et al. 2020, Ghezelayagh et al. 2021; Islam et al. 2021; Madani et al. 2021; Xaki et al. 2021; Aghayan et al. 2022). Nowadays, this fact has been discovered that MSC therapy may inhibit the active immune system from triggering a cytokine storm in COVID-19 cases, and the stem cells' reparative powers may support endogenous repair (Ellison-Hughes et al. 2020; Parhizkar Roudsari et al. 2020; Rajarshi et al. 2020; Arjmand et al. 2021, Gilany et al. 2021). MSCs become activated and adopt an immune-suppressive phenotype in the presence of an inflammatory environment (high levels of TNF- and IFN-), by secreting high levels of soluble factors such as indoleamine 2, 3-dioxygenase (IDO), prostaglandin E2 (PGE2), nitric oxide (NO), transforming growth factor (TGF-β), hepatocyte growth factor (HGF), and hemoxygenase (HO), which suppress T cell proliferation. Moreover, the development of regulatory T cells (Tregs) is promoted by MSCs' constant production of TGF-β (Zheng et al. 2015; Contreras et al. 2016; Ellison-Hughes et al. 2020). However, MSCs may develop a pro-inflammatory phenotype and boost T cell responses by secreting chemokines that attract lymphocytes to sites of inflammation (i.e., MIP-, RANTES, C-X-C Motif Chemokine Ligand (CXCL)9, and CXCL10) in the absence of an inflammatory environment (low levels of TNF- and IFN-). The difficult balancing act between these opposing routes might strengthen the host defense while also encouraging regeneration (Meiliana et al. 2016; Ellison-Hughes et al. 2020; Mishra et al. 2020). On the other hand, MSCs aggregate in the lungs, where they can reduce or prevent lung fibrosis by having anti-inflammatory effects, improving the lung microenvironment, and possibly restoring vascular barrier integrity, as well as promoting endogenous repair and regeneration mechanisms (Ellison-Hughes et al. 2020). Furthermore, the antimicrobial capabilities of MSCs have been demonstrated. Hereupon, based on their predominantly immunosuppressive characteristics, MSCs' impact on host defense against a live bacterial infection has been questioned. They can improve survival and reduce bacterial numbers in the blood. In this context, MSCs' ability to upregulate antimicrobial peptides (Lipocalin 2) production in response to inflammatory stimuli such as LPS and TNFα contributes to the bacterial clearance effect observed with MSC therapeutic interventions. Generally, scientists found that systemic treatment with MSC activation could be a promising new strategy for treating multidrug-resistant bacterium infections (Krasnodembskaya et al. 2010; Gupta et al. 2012; Johnson et al. 2017; Mishra et al. 2020; Yagi et al. 2020). Figure 4 shows the different effects of MSCs that contribute to their therapeutic function. Generally, the safety and potential effectiveness of any novel therapeutic intervention are both heavily dependent on preclinical research. Accordingly, an early assessment of MSCs' preclinical performance is necessary before using them in the COVID-19 clinical phase. Nevertheless, due to the rapid spread of the COVID-19 pandemic, studies have centered more on clinical rather than preclinical studies. But, some preclinical experiments in animal models of ARDS and lung injury have shown that MSCs intervention may be advantageous and a number of animal models that resemble the pathophysiology of COVID-19 have been identified (e.g., hamsters, Mice, Ferret, mink, cats, dogs and non-human primates) and others are still being developed. Indeed, since SARS-CoV-2 is a recently discovered virus, it has to be more studied (El-Metwaly et al. 2019, Jung et al. 2019; Rahmati et al. 2020; Saleh and Ghazzawi 2021; Shou et al. 2021). Therefore, majority of investigations in the field of Covid-19 have been in the clinical stage. Herein Table 2 shows clinical trials based on MSCs in COVID-19.
Therapeutic effects of mesenchymal stem cells. Mesenchymal stem cells can play a fundamental role in COVID-19 treatment via realizing several angiogenic, mitogenic, anti-apoptotic, anti-inflammatory, and anti-oxidative factors. They are implicated in the down-regulation of acute-phase response, such as the suppression of inappropriately activated T lymphocytes, and macrophages, and the release of pro-inflammatory cytokines, which could minimize the occurrence of cytokine storms (immunomodulatory effects. Furthermore, they have the ability to suppress cell apoptosis (anti-apoptotic effects), enhance endogenous tissue repair (regenerative effects), and release antimicrobial compounds (antimicrobial effects). Direct scavenging of free radicals, promoting endogenous antioxidant defenses, immunomodulation via reactive oxygen species (ROS) suppression, altering mitochondrial bioenergetics, and donating functional mitochondria to damaged cells are some of the mechanisms by which mesenchymal stem cells have antioxidant effects. Moreover, mesenchymal stem cells can enhance angiogenesis through direct differentiation, cell–cell contact, or paracrine actions (Sadeghi et al. 2020; Fernández-Francos et al. 2021). BioRender was used to create this image. Indoleamine 2, 3-dioxygenase (IDO), Interferon gamma (IFN-γ), Prostaglandin E2 (PGE2), Tumor necrosis factor α (TNFα), Interleukins (IL), Transforming growth factor beta (TGF-β), C–X–C motif chemokine (CXCL), Induced protein 10 (IP-10), C‑C motif chemokine ligand 5 (CCL5), Lipocalin-2 (LCN2), Fibroblast growth factor (FGF), Insulin-like growth factor 1 (IGF-1), Granulocyte–macrophage colony-stimulating factor (GM-CSF), Hepatocyte growth factor (HGF), Tissue inhibitors of matrix metalloproteinases (TIMP), Vascular endothelial growth factor (VEGF), Platelet-derived growth factor (PDGF), Epidermal growth factor (EGF), Angiopoietin (Ang), Monocyte chemoattractant protein-1 (MCP-1), Matrix metalloproteinases (MMPs), Superoxide dismutase (SOD), Catalase (CAT), Glutathione peroxidases (GPXs), Glutathione (GSH), Heat shock protein 70 (Hsp70)
Mesenchymal stem cells-based vs. cell-free strategies (limitations and advantages)
According to previous paragraphs, the therapeutic potential of MSCs as a subgroup of stem cells is considerably investigated. Also, unlike other types of stem cells, the source of MSCs is from various tissues which dedicate this subtype a wide availability. Additionally, many studies and clinical trials have investigated MSCs' therapeutic role in COVID-19. In this regard, one of the main pathogenesis in severe COVID patients is hyper inflammation which is hypothesized that MSC and its derivatives may suppress it through their anti-inflammatory and immunomodulatory properties (Leng et al. 2020). Additionally, studies have shown that MSCs through ceasing the maturation process of several types of immune cells and promoting regulatory T-cells suppress the immune system (Sarvar et al. 2016). Moreover, MSCs mediate their regenerative, immunomodulatory, anti-tumor, and other effects on the other cells and tissues through secreting EVs, cytokines, chemokine, soluble proteins, and many other paracrine factors. These factors and EVs have the privilege of altering their contents and even determining their target by editing their receptors. Also, EVs play a pivotal role in intra- and inter-cellular interactions. Hence, EVs could be administered as vehicles to transfer drugs and special substances including genes to target cells and tissues (Rezakhani et al. 2021). Thus, supplying a conditioned medium including these factors and EVs could dominate many problems of applying MSCs per se. In this regard, tumor differentiation, uncontrolled migration, transplant rejection and etc. are some of the disadvantages of cell-based therapies that are not or less seen in cell-free approaches. As well as, EVs have smaller sizes in proportion to their source cells which reduces the risk of thrombosis and pulmonary embolization. Totally, cell-free therapeutics are safer with long-lasting storage in comparison to cell-based tools. Also, due to regulatory restrictions, MSCs-based products and cell-free approaches with less or no stem cell manipulation shed light on the future of the RM era (Sagaradze et al. 2018; Rahmati et al. 2020; Wang et al. 2021; Sharun et al. 2022). However, reaching the standard protocol to apply MSCs-based products still needs further studies and consideration.
Conclusion and future perspectives
Recently, the need for effective therapies in the face of COVID-19 and its important consequences such as CRS and co- bacterial infections has intensified. In this respect, stem cell therapy and stem cell-based organoid models are gaining popularity as new treatment and research tools for COVID-19. Especially, MSC-based therapies have lately piqued scientists' interest due to their potential utility in autologous transplantation. Generally, over the years, great progress has been made in understanding the potential of MSCs and there is a good foundation for future scientific research and therapeutic applications. It's also crucial to take into account the fact that MSCs produced from various tissue sources have phenotypic heterogeneity, exhibit various potential for differentiation, and release various physiologically active substances. Therefore, choosing sources of MSCs with certain biological characteristics will support the development of precision medicines in the future. The best source of cells for really ill elderly COVID-19 patients are allogeneic cells and autologous donors can be used, for younger patients. Generally, to treat COVID-19, a trustworthy source of MSCs must be identified. Regulations from authorities and clinical guidelines are required since MSC suppliers have different quality criteria and researchers give MSC products different clinical grades. Accordingly, several experts have already provided therapeutic recommendations for MSC COVID-19 medicines in an effort to reach international consensus (Zumla et al. 2020; Ruonan et al. 2021). On the other hand, finding altered signaling pathways during viral infections focusing on inflammatory, oxidative stress, apoptotic, and autophagic pathways could help decipher the most important molecular cascades involved in biological processes driving viral infections, as well as identify key molecular players who could be targeted. Hereupon, therapeutic strategies targeted at simultaneously inhibiting all pro-inflammatory cytokine and chemokine pathways are presented in the context of COVID-19. Herein, in critical stage research, simultaneous suppression of numerous cytokines/chemokine is believed to offer far greater therapeutic potential than single target techniques to prevent the cascade effects of numerous elicited cytokines and chemokine in COVID-19 patients. Additionally, more studies are required to develop an effective delivery system that overcomes the limits of therapeutic interventions in COVID-19 along with laying the stage for the eventual creation of instruments that can precisely track COVID-19 patient trajectories using information from metabolic pathways. Generally, in any COVID-19 pandemic future scenario, how civilizations react will play a significant role. Beginning with the extent to which nations can efficiently scale and make available new therapies with the potential to lower the likelihood of progression to severe disease, some levers are expected to be especially crucial. In this context, faster distribution of booster doses will assist safeguard the population and finding the ideal mix of public-health initiatives will be crucial given public stress and the lessons of the previous years of getting a disease.
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- ACE2:
-
Angiotensin-Converting Enzyme 2
- ARDS:
-
Acute Respiratory Distress Syndrome
- CAT:
-
Catalase
- CCL5:
-
C‑C motif Chemokine Ligand 5
- COPD:
-
Chronic Obstructive Pulmonary Disease
- COVID-19:
-
Coronavirus Disease 2019
- CRS:
-
Cytokine Release Storm
- CXCL:
-
C-X-C motif Chemokine Ligand
- DAMPs:
-
Damage Associated Molecular Patterns
- DCs:
-
Dendritic Cells
- EGF:
-
Epidermal Growth Factor
- ERK:
-
Extracellular signal Regulated Kinase
- EVs:
-
Extracellular Vesicles
- FDA:
-
Food and Drug Administration
- FGF:
-
Fibroblast Growth Factor
- GH:
-
Growth Hormone
- GM-CSF:
-
Granulocyte Macrophage Colony Stimulating Factor
- GPx:
-
Glutathione Peroxidases
- GSH:
-
Glutathione
- HGF:
-
Hepatocyte Growth Factor
- HSP70:
-
Heat shock protein 70
- IDO:
-
Indoleamine 2, 3-dioxygenase
- IFN:
-
Interferon
- IGF-1:
-
Insulin-like Growth Factor-1
- IL:
-
Interleukin
- JAK-STAT:
-
Janus Kinase -Signal Transducer and Activator of Transcription
- JNK:
-
Jun NH (2)-terminal Kinase
- LCN2:
-
Lipocalin-2
- MAPK:
-
Mitogen Activated Protein Kinase
- MCP-1:
-
Monocyte Chemoattractant Protein-1
- MMPs:
-
Matrix Metalloproteinases
- MSCs:
-
Mesenchymal Stem Cells
- NF-κB:
-
Nuclear Factor kappa B
- NK:
-
Natural Killer
- NLRP3:
-
Nucleotide-binding oligomerization domain-like receptors containing pyrin domain 3
- NLRs:
-
Nucleotide-binding oligomerization domain-like receptors
- PAMPs:
-
Pathogen Associated Molecular Patterns
- PDGF:
-
Platelet Derived Growth Factors
- PGE2:
-
Prostaglandin E2
- PTPs:
-
Protein Tyrosine Phosphatases
- RA:
-
Rheumatoid Arthritis
- RAAS:
-
Renin Angiotensin Aldosterone System
- RM:
-
Regenerative Medicine
- SAR:
-
Sarilumab
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- SOCS:
-
Suppressor of Cytokine Signaling
- SOD:
-
Superoxide Dismutase
- TCZ:
-
Tocilizumab
- TNF:
-
Tumor Necrosis Factor
- TGF-β:
-
Transforming Growth Factor-β
- TIMP:
-
Tissue Inhibitors of Matrix metalloproteinases
- TLRs:
-
Toll like Receptors
- TMPRSS2:
-
Transmembrane protease serine 2
- TTSPs:
-
Type-II Transmembrane Serine Proteases
- Tyk2:
-
Tyrosine kinase 2
- VAP:
-
Ventilator-Associated Pneumonia
- VEGF:
-
Vascular Endothelial Growth Factor
References
Abedi M, Alavi-Moghadam S, Payab M, Goodarzi P, Mohamadi-Jahani F, Sayahpour FA, Larijani B, Arjmand B (2020) Mesenchymal stem cell as a novel approach to systemic sclerosis; current status and future perspectives. Cell Regeneration 9(1):1–19
Aghayan HR, Hosseini MS, Gholami M, Mohamadi-Jahani F, Tayanloo-Beik A, Alavi-Moghadam S, Payab M, Goodarzi P, Abdollahi M, Larijani B (2022) Mesenchymal stem cells’ seeded amniotic membrane as a tissue-engineered dressing for wound healing. Drug Deliv Transl Res 12(3):538–549
Aghayan HR, Payab M, Mohamadi-Jahani F, Aghayan SS, Larijani B, Arjmand B (2020) GMP-compliant production of human placenta-derived mesenchymal stem cells. Stem cells and good manufacturing practices. Springer, pp 213–225
Aghili SMM, Ebrahimpur M, Arjmand B, Shadman Z, Pejman Sani M, Qorbani M, Larijani B, Payab M (2021) Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta-analysis. Int J Obes 45(5):998–1016
Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O’Callaghan A, Pardos-Gea J, Quintana A, Mekinian A, Anunciacion-Llunell A, Miró-Mur F (2020) Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: a comprehensive review. Autoimmun Rev 19(7):102569–102569
Alrubayyi A (2020) NK cells in COVID-19: protectors or opponents? Nat Rev Immunol 20(9):520–520
Arjmand B, Alavi-Moghadam S, Parhizkar Roudsari P, Rezaei-Tavirani M, Rahim F, Gilany K, Mohamadi-Jahani F, Adibi H, Larijani B (2021) COVID-19 pathology on various organs and regenerative medicine and stem cell-based interventions. Front Cell Dev Biol 9:1342
Arjmand B, Ghorbani F, Koushki M, Rezai-Tavirani M (2020a) Gastrointestinal symptoms in patients with mild and severe COVID-19: a scoping review and meta-analysis. Gastroenterol Hepatol Bed Bench 13(4):321
Arjmand B, Goodarzi P, Aghayan HR, Payab M, Rahim F, Alavi-Moghadam S, Mohamadi-Jahani F, Larijani B (2019) Co-transplantation of human fetal mesenchymal and hematopoietic stem cells in type 1 diabetic mice model. Front Endocrinol 10:761
Arjmand B, Sarvari M, Alavi-Moghadam S, Payab M, Goodarzi P, Gilany K, Mehrdad N, Larijani B (2020b) Prospect of stem cell therapy and regenerative medicine in osteoporosis. Front Endocrinol 11:430
Auletta JJ, Deans RJ, Bartholomew AM (2012) Emerging roles for multipotent, bone marrow-derived stromal cells in host defense. Blood 119(8):1801–1809
Azodi MZ, Arjmand B, Razzaghi M, Tavirani MR, Ahmadzadeh A, Rostaminejad M (2021) Platelet and haemostasis are the main targets in severe cases of COVID-19 infection; a system bioinformatics study. Arch Acad Emergency Med 9(1):e27–e27
Azodi MZ, Arjmand B, Zali A, Razzaghi M (2020) Introducing APOA1 as a key protein in COVID-19 infection: a bioinformatics approach. Gastroenterol Hepatol Bed Bench 13(4):367
Batiha GE, Al-Gareeb DAI, Qusti S, Alshammari EM, Rotimi D, Adeyemi OS, Al-Kuraishy HM (2021) Common NLRP3 inflammasome inhibitors and Covid-19: divide and conquer. Sci Afr. https://doi.org/10.1016/j.sciaf.2021.e01084
Batu ED, Özen S (2020) Implications of COVID-19 in pediatric rheumatology. Rheumatol Int 40(8):1193–1213
Bousoik E, Montazeri Aliabadi H (2018) “Do We Know Jack” About JAK? A closer look at JAK/STAT signaling pathway. Front Oncol. https://doi.org/10.3389/fonc.2018.00287
Braun E, Sauter D (2019) Furin-mediated protein processing in infectious diseases and cancer. Clin Transl Immunol 8(8):e1073–e1073
Breikaa RM, Lilly B (2021) The notch pathway: a link between COVID-19 pathophysiology and its cardiovascular complications. Front Cardiovasc Med 8:681948–681948
Carsetti R, Zaffina S, Piano Mortari E, Terreri S, Corrente F, Capponi C, Palomba P, Mirabella M, Cascioli S, Palange P (2020) Different innate and adaptive immune responses to SARS-CoV-2 infection of asymptomatic, mild, and severe cases. Front Immunol 11:3365
Chaplin DD (2010) Overview of the immune response. J Allergy Clin Immunol 125(2 Suppl 2):S3–S23
Chaudhry R, Sreenath K, Batra P, Vinayaraj E, Rathor N, Saikiran K, Aravindan A, Singh V, Brijwal M, Soneja M (2021) Atypical bacterial co-infections among patients with COVID-19: a study from India. J Med Virol 94(1):303–309
Chen J, Subbarao K (2007) The immunobiology of SARS. Annu Rev Immunol 25:443–472
Chertow DS, Memoli MJ (2013) Bacterial coinfection in influenza: a grand rounds review. JAMA 309(3):275–282
Chi P-I, Liu H-J (2013) Molecular signaling and cellular pathways for virus entry. ISRN Virol 2013:306595
Chowdhury MA, Hossain N, Kashem MA, Shahid MA, Alam A (2020) Immune response in COVID-19: a review. J Infect Public Health 13(11):1619–1629
Contreras RA, Figueroa FE, Djouad F, Luz-Crawford P (2016) Mesenchymal stem cells regulate the innate and adaptive immune responses dampening arthritis progression. Stem Cells Int 2016:3162743–3162743
Cruz FF, Rocco PRM (2020) The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev Respir Med 14(1):31–39
Cuenda A (2019) Mitogen-activated protein kinases (MAPK) in cancer. In: Boffetta P, Hainaut P (eds) Encyclopedia of cancer, 3rd edn. Academic Press, Oxford, pp 472–480
Dandekar AA, Perlman S (2005) Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 5(12):917–927
Dayer MR (2021) New Candidates for Furin Inhibition as Probable Treat for COVID-19: Docking Output. arXiv preprint arXiv:2107.12799
de Queiroz TM, Lakkappa N, Lazartigues E (2020) ADAM17-mediated shedding of inflammatory cytokines in hypertension. Front Pharmacol. https://doi.org/10.3389/fphar.2020.01154
El-Metwaly S, El-Senduny FF, El-Demerdash RS, Abdel-Aziz A (2019) Mesenchymal stem cells alleviate hydrochloric acid-induced lung injury through suppression of inflammation, oxidative stress and apoptosis in comparison to moxifloxacin and sildenafil. Heliyon 5(12):e02710
Ellison-Hughes GM, Colley L, O’Brien KA, Roberts KA, Agbaedeng TA, Ross MD (2020) The role of MSC therapy in attenuating the damaging effects of the cytokine storm induced by COVID-19 on the heart and cardiovascular system. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2020.602183
Esposito D, Schaumann D, Camarda D, Kalay YE (2020) Multi-agent modelling and simulation of hospital acquired infection propagation dynamics by contact transmission in hospital wards. In: International Conference on Practical Applications of Agents and Multi-Agent Systems, Springer
Farahani M, Niknam Z, Mohammadi Amirabad L, Amiri-Dashatan N, Koushki M, Nemati M, Danesh Pouya F, Rezaei-Tavirani M, Rasmi Y, Tayebi L (2022) Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. Biomed Pharmacother 145:112420
Farrell JM, Zhao CY, Tarquinio KM, Brown SP (2021) Causes and consequences of COVID-19-associated bacterial infections. Front Microbiol 12:682571
Fattorini L, Creti R, Palma C, Pantosti A (2020) Bacterial coinfections in COVID-19: an underestimated adversary. Annali Dell’istituto Superiore Di Sanita 56(3):359–364
Feldman C, Anderson R (2021) The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 13(1):1–15
Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F (2020) COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med 201(11):1380–1388
Fernández-Francos S, Eiro N, Costa LA, Escudero-Cernuda S, Fernández-Sánchez ML, Vizoso FJ (2021) Mesenchymal stem cells as a cornerstone in a galaxy of intercellular signals: basis for a new era of medicine. Int J Mol Sci 22(7):3576
Földvári-Nagy L, Schnabel T, Dörnyei G, Korcsmáros T, Lenti K (2021) On the role of bacterial metalloproteases in COVID-19 associated cytokine storm. Cell Commun Signal 19(1):7–7
Franchi L, McDonald C, Kanneganti TD, Amer A, Núñez G (2006) Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol 177(6):3507–3513
Fu Y, Yang Q, Xu M, Kong H, Chen H, Fu Y, Yao Y, Zhou H, Zhou J (2020) Secondary bacterial infections in critical ill patients of COVID-19. Open Forum Infect Dis. https://doi.org/10.1093/ofid/ofaa220
Furuhashi M, Moniwa N, Takizawa H, Ura N, Shimamoto K (2020) Potential differential effects of renin-angiotensin system inhibitors on SARS-CoV-2 infection and lung injury in COVID-19. Hypertens Res 43(8):837–840
García LF (2020) Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol 11:1441
Gelmann EP, Sawyers CL, Rauscher FJ III (2013) Molecular oncology: causes of cancer and targets for treatment. Cambridge University Press
Ghandour M, Suleiman S, Osman-Malik Y, Bhat ZY (2021) Remdesivir induced liver injury in a patient with Coronavirus Disease 2019. Am J Medical Case Rep 9(3):170–171
Ghasemzadeh M, Hosseini E, Ghasemzadeh A (2021) Exhausted NK cells and cytokine storms in COVID-19: whether NK cell therapy could be a therapeutic choice. Hum Immunol 83(1):86–98
Ghazanfar H, Kandhi S, Shin D, Muthumanickam A, Gurjar H, Qureshi ZA, Shaban M, Farag M, Haider A, Budhathoki P, Bhatt T, Ghazanfar A, Jyala A, Patel H (2022) Impact of COVID-19 on the gastrointestinal tract: a clinical review. Cureus 14(3):e23333
Ghezelayagh Z, Zabihi M, Zarkesh I, Gonçalves CA, Larsen M, Hagh-Parast N, Pakzad M, Vosough M, Arjmand B, Baharvand H (2021) Improved differentiation of hESC-derived pancreatic progenitors by using human fetal pancreatic mesenchymal cells in a micro-scalable three-dimensional co-culture system. Stem Cell Rev Rep 18(1):360–377
Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami M-E, Katsaounou P (2020) Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27(6):992–1000
Gilany K, Goodarzi P, Tayanloo-Beik A, Masroor MJ, Mani-Varnosfaderani A, Rezaei-Tavirani M, Aghayan H, Kordi R, Arjmand B, Larijani B (2021) Looking at time dependent differentiation of mesenchymal stem cells by culture media using MALDI-TOF-MS. Cell Tissue Banking 23(4):653–668
Golchin A, Farahany TZ (2019) Biological products: cellular therapy and FDA approved products. Stem Cell Rev Rep 15(2):166–175
Golchin A, Seyedjafari E, Ardeshirylajimi A (2020) Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep 16(3):427–433
Goncalves Mendes Neto A, Lo KB, Wattoo A, Salacup G, Pelayo J, DeJoy R III, Bhargav R, Gul F, Peterson E, Albano J (2021) Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol 93(3):1489–1495
Goodarzi P, Alavi-Moghadam S, Payab M, Larijani B, Rahim F, Gilany K, Bana N, Tayanloo-Beik A, Heravani NF, Hadavandkhani M (2019a) Metabolomics analysis of mesenchymal stem cells. Int J Mol Cell Med 8(Suppl1):30
Goodarzi P, Falahzadeh K, Aghayan H, Payab M, Larijani B, Alavi-Moghadam S, Tayanloo-Beik A, Adibi H, Gilany K, Arjmand B (2019b) Therapeutic abortion and ectopic pregnancy: alternative sources for fetal stem cell research and therapy in Iran as an Islamic country. Cell Tissue Banking 20(1):11–24
Goodarzi P, Larijani B, Alavi-Moghadam S, Tayanloo-Beik A, Mohamadi-Jahani F, Ranjbaran N, Payab M, Falahzadeh K, Mousavi M, Arjmand B (2018) Mesenchymal stem cells-derived exosomes for wound regeneration. Cell Biol Transl Med 4:119–131
Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA (2012) Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 67(6):533–539
Hamel M, Zoutman D, O’Callaghan C (2010) Exposure to hospital roommates as a risk factor for health care–associated infection. Am J Infect Control 38(3):173–181
Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y (2020) Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 9(1):1123–1130
Hariharan A, Hakeem AR (2021) The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology 29(1):91–100
Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, Nguyen GH, Le PTT, Hoang VT, Forsyth NR, Heke M, Nguyen LT (2022) Stem cell-based therapy for human diseases. Signal Transduct Target Ther 7(1):272
Hocková B, Riad A, Valky J, Šulajová Z, Stebel A, Slávik R, Bečková Z, Pokorná A, Klugarová J, Klugar M (2021) Oral complications of ICU patients with COVID-19: case-series and review of two hundred ten cases. J Clin Med 10(4):581
Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, Hirano T (2020) How COVID-19 induces cytokine storm with high mortality. Inflammation Regeneration 40(1):1–7
Horiuchi M, Akishita M, Dzau VJ (1999) Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 33(2):613–621
Hu X, Li J, Fu M, Zhao X, Wang W (2021) The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Targeted Ther 6(1):402
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395(10223):497–506
Huang Y-Q (2015) Application of mesenchymal stem cells in digestive system diseases. World Chin J Digestol 23:2688
Isaacs JD, Burmester GR (2020) Smart battles: immunosuppression versus immunomodulation in the inflammatory RMDs. BMJ Publishing Group Ltd
Islam MS, Al Mahtab M, Shirian S, Aghayan HR, Arjmand B, Allahverdi A, Ranjbar FE, Sadegh AB, Ai J (2021) Encapsulation of rat bone marrow derived mesenchymal stem cells (rBMMSCs) in collagen type i containing platelet-rich plasma for osteoarthritis treatment in rat model. Prog Biomater 11(4):385–396
Johnson V, Webb T, Norman A, Coy J, Kurihara J, Regan D, Dow S (2017) Activated mesenchymal stem cells interact with antibiotics and host innate immune responses to control chronic bacterial infections. Sci Rep 7(1):1–18
Jung YJ, Park YY, Huh JW, Hong S-B (2019) The effect of human adipose-derived stem cells on lipopolysaccharide-induced acute respiratory distress syndrome in mice. Ann Transl Med 7(22):674
Klimpel GR (1996) Immune defenses. In: Medical microbiology, 4th edn. The University of Texas Medical Branch, Galveston
Krämer OH, Heinzel T (2010) Phosphorylation–acetylation switch in the regulation of STAT1 signaling. Mol Cell Endocrinol 315(1–2):40–48
Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA (2010) Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 28(12):2229–2238
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med 11(8):875–879
Kumar R, Khandelwal N, Thachamvally R, Tripathi BN, Barua S, Kashyap SK, Maherchandani S, Kumar N (2018) Role of MAPK/MNK1 signaling in virus replication. Virus Res 253:48–61
Lan T, Luo M, Wei X (2021) Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol 14(1):1–16
Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, Soucy J-PR, Daneman N (2020) Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 26(12):1622–1629
Lansbury L, Lim B, Baskaran V, Lim WS (2020) Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect 81(2):266–275
Larijani B, Aghayan H-R, Goodarzi P, Arjmand B (2014) GMP-grade human fetal liver-derived mesenchymal stem cells for clinical transplantation. Stem cells and good manufacturing practices. Springer, pp 123–136
Larijani B, Aghayan H, Goodarzi P, Mohamadi-Jahani F, Norouzi-Javidan A, Dehpour AR, Fallahzadeh K, Sayahpour FA, Bidaki K, Arjmand B (2015) Clinical grade human adipose tissue-derived mesenchymal stem cell banking. Acta Med Iran 53(9):540–546
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S (2020) Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 11(2):216
León-Rodríguez SGD, Hernández-Rico B, Olmo-Vázquez GD, Cruz-Dávalos I, Bonifaz LC (2020) SARS-CoV-2: previous coronaviruses, immune response, and development of vaccines. Bol Med Hosp Infant Mex 77(5):252–261
Li W, Qiao J, You Q, Zong S, Peng Q, Liu Y, Hu S, Liu W, Li S, Shu X, Sun B (2021) SARS-CoV-2 Nsp5 activates NF-κB pathway by upregulating SUMOylation of MAVS. Front Immunol. https://doi.org/10.3389/fimmu.2021.7509
Luo J, Lu S, Yu M, Zhu L, Zhu C, Li C, Fang J, Zhu X, Wang X (2021) The potential involvement of JAK-STAT signaling pathway in the COVID-19 infection assisted by ACE2. Gene 768:145325
Madani S, Setudeh A, Aghayan HR, Alavi-Moghadam S, Rouhifard M, Rezaei N, Rostami P, Mohsenipour R, Amirkashani D, Bandarian F (2021) Placenta derived Mesenchymal Stem Cells transplantation in Type 1 diabetes: preliminary report of phase 1 clinical trial. J Diabetes Metab Disord 20(2):1179–1189
Maes M, Del Tedesco Junior WL, Lozovoy MAB, Mori MTE, Danellii T, Delicato de Almeida ER, Tejo AM, Tano ZN, Reiche EMV, Simão ANC (2021) In COVID-19, <em>NLRP3</em> inflammasome genetic variants are associated with critical disease and these effects are partly mediated by the sickness symptom complex: a nomothetic network approach. medRxiv: 2021.2009.2026.21264127
Mahmoudi H (2020) Bacterial co-infections and antibiotic resistance in patients with COVID-19. GMS Hygiene Infect Control. https://doi.org/10.3205/dgkh000370
Mao AS, Mooney DJ (2015) Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci 112(47):14452–14459
Marc F, Moldovan CM, Hoza A, Magheru S, Ciavoi G, Farcas DM, Sachelarie L, Calin G, Romila L, Damir D (2022) Comparative study of cytokine storm treatment in patients with COVID-19 pneumonia using immunomodulators. J Clin Med 11(10):2945
Meiliana A, Dewi NM, Wijaya A (2016) Mesenchymal stem cells manage endogenous tissue regeneration. Indonesian Biomed J 8(2):71–90
Mishra VK, Shih H-H, Parveen F, Lenzen D, Ito E, Chan T-F, Ke L-Y (2020) Identifying the therapeutic significance of mesenchymal stem cells. Cells 9(5):1145
Moezzi M, Shirbandi K, Shahvandi HK, Arjmand B, Rahim F (2021) The diagnostic accuracy of Artificial Intelligence-Assisted CT imaging in COVID-19 disease: a systematic review and meta-analysis. Inform Med Unlocked 24:100591
Niknam Z, Jafari A, Golchin A, Danesh Pouya F, Nemati M, Rezaei-Tavirani M, Rasmi Y (2022) Potential therapeutic options for COVID-19: an update on current evidence. Eur J Med Res 27(1):6
Oeckinghaus A, Ghosh S (2009) The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1(4):a000034–a000034
Orilisi G, Mascitti M, Togni L, Monterubbianesi R, Tosco V, Vitiello F, Santarelli A, Putignano A, Orsini G (2021) Oral manifestations of COVID-19 in hospitalized patients: a systematic review. Int J Environ Res Public Health 18(23):12511
Parhizkar Roudsari P, Alavi-Moghadam S, Payab M, Sayahpour FA, Aghayan HR, Goodarzi P, Mohamadi-Jahani F, Larijani B, Arjmand B (2020) Auxiliary role of mesenchymal stem cells as regenerative medicine soldiers to attenuate inflammatory processes of severe acute respiratory infections caused by COVID-19. Cell Tissue Banking 21(3):405–425
Pourajam S, Kalantari E, Talebzadeh H, Mellali H, Sami R, Soltaninejad F, Amra B, Sajadi M, Alenaseri M, Kalantari F (2022) Secondary bacterial infection and clinical characteristics in patients with COVID-19 admitted to two intensive care units of an academic hospital in iran during the first wave of the pandemic. Front Cell Infect Microbiol 12:784130
Póvoa HCC, Chianca GC, Iorio NLPP (2020) COVID-19: an alert to ventilator-associated bacterial pneumonia. Infect Dis Ther 9:417–420
Racaniello V, Tuller D, Rey G (2020) Furin cleavage site in the SARS-CoV-2 coronavirus glycoprotein. Virology Blog
Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R (2020) The COVID-19 cytokine storm; what we know so far. Front Immunol. https://doi.org/10.3389/fimmu.2020.0144
Rahmani-Kukia N, Abbasi A, Froushani SMA, Shahgaldi S, Mokarram P (2020) The effects of 17 Beta-Estradiol primed mesenchymal stem cells on the biology of co-cultured neutrophil. Int Immunopharmacol 84:106602
Rahmati S, Shojaei F, Shojaeian A, Rezakhani L, Dehkordi MB (2020) An overview of current knowledge in biological functions and potential theragnostic applications of exosomes. Chem Phys Lipid 226:104836
Rajarshi K, Chatterjee A, Ray S (2020) Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol Rep 26:e00467
Rana MM (2020) Cytokine storm in COVID-19: potential therapeutics for immunomodulation. J Res Clin Med 8(1):38–38
Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, Satta G, Cooke G, Holmes A (2020) Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 71(9):2459–2468
Rezakhani L, Kelishadrokhi AF, Soleimanizadeh A, Rahmati S (2021) Mesenchymal stem cell (MSC)-derived exosomes as a cell-free therapy for patients Infected with COVID-19: real opportunities and range of promises. Chem Phys Lipid 234:105009
Rezk NA, Ibrahim AM (2013) Effects of methyl prednisolone in early ARDS. Egyptian J Chest Dis Tuberculosis 62(1):167–172
Rice TW, Rubinson L, Uyeki TM, Vaughn FL, John BB, Miller RR III, Higgs E, Randolph AG, Smoot BE, Thompson BT (2012) Critical illness from 2009 pandemic influenza A (H1N1) virus and bacterial co-infection in the United States. Crit Care Med 40(5):1487
Rivera-Cruz CM, Shearer JJ, Figueiredo Neto M, Figueiredo ML (2017) The immunomodulatory effects of mesenchymal stem cell polarization within the tumor microenvironment niche. Stem Cells Int 2017:4015039
Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R (2020) COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Springer 115:1–8
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68(2):320–344
Ruonan X, Lei S, Wei-Fen X, Zhe X, Fan-Ping M, Jun-Liang F, Xin Y, Lei H, Ming S, Yonggang L (2021) Diagnosis and treatment guidelines for mesenchymal stem cell therapy for coronavirus disease 2019 (Beijing, 2021). Infect Dis Immun 1(02):68–73
Sadeghi S, Soudi S, Shafiee A, Hashemi SM (2020) Mesenchymal stem cell therapies for COVID-19: Current status and mechanism of action. Life Sci 262:118493
Saeedi P, Halabian R, Fooladi AAI (2019) Antimicrobial effects of mesenchymal stem cells primed by modified LPS on bacterial clearance in sepsis. J Cell Physiol 234(4):4970–4986
Sagaradze GD, Nimiritsky PP, Akopyan ZA, Makarevich PI, Efimenko AY (2018) Cell-free therapeutics” from components secreted by mesenchymal stromal cells as a novel class of biopharmaceuticals. Biopharmaceuticals 2:47–61
Sahu T, Mehta A, Ratre YK, Jaiswal A, Vishvakarma NK, Bhaskar L, Verma HK (2021) Current understanding of the impact of COVID-19 on gastrointestinal disease: challenges and openings. World J Gastroenterol 27(6):449–469
Saleh FA, Ghazzawi J (2021) Clinical update on the use of mesenchymal stem cells in COVID-19. Am J Transl Res 13(11):12195
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19): a review. JAMA 323(18):1824–1836
Sant AJ, McMichael A (2012) Revealing the role of CD4+ T cells in viral immunity. J Exp Med 209(8):1391–1395
Sarvar DP, Shamsasenjan K, Akbarzadehlaleh P (2016) Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv Pharmaceutical Bull 6(3):293
Satarker S, Tom AA, Shaji RA, Alosious A, Luvis M, Nampoothiri M (2021) JAK-STAT pathway inhibition and their implications in COVID-19 therapy. Postgrad Med 133(5):489–507
Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M (2017) The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal 15(1):1–13
Sharifipour E, Shams S, Esmkhani M, Khodadadi J, Fotouhi-Ardakani R, Koohpaei A, Doosti Z, Golzari SE (2020) Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis 20(1):1–7
Sharun K, Dhama K, Jambagi K, Pawde AM, Amarpal (2022). Cell-free therapy for inflammatory diseases: opportunities and challenges. Recent Adv Inflamm Allergy Drug Discov 15(1): 5–8
Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z (2020) Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 368(6494):1016–1020
Shou S, Liu M, Yang Y, Kang N, Song Y, Tan D, Liu N, Wang F, Liu J, Xie Y (2021) Animal models for COVID-19: hamsters, mouse, ferret, mink, tree shrew, and non-human primates. Front Microbiol 12:626553
Shoukat A, Wells CR, Langley JM, Singer BH, Galvani AP, Moghadas SM (2020) Projecting demand for critical care beds during COVID-19 outbreaks in Canada. CMAJ 192(19):E489–E496
Sinha P, Matthay MA, Calfee CS (2020) Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med 180(9):1152–1154
Sirivongrangson P, Kulvichit W, Payungporn S, Pisitkun T, Chindamporn A, Peerapornratana S, Pisitkun P, Chitcharoen S, Sawaswong V, Worasilchai N, Kampunya S, Putcharoen O, Thawitsri T, Leelayuwatanakul N, Kongpolprom N, Phoophiboon V, Sriprasart T, Samransamruajkit R, Tungsanga S, Tiankanon K, Lumlertgul N, Leelahavanichkul A, Sriphojanart T, Tantawichien T, Thisyakorn U, Chirathaworn C, Praditpornsilpa K, Tungsanga K, Eiam-Ong S, Sitprija V, Kellum JA, Srisawat N (2020) Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensive Care Med Exp 8(1):72
Soleimanian S, Yaghobi R (2020) Harnessing memory NK cell to protect against COVID-19. Front Pharmacol. https://doi.org/10.3389/fphar.2020.01309
Song J, Kang S, Choi SW, Seo KW, Lee S, So MW, Lim D-H (2020) Coronavirus Disease 19 (COVID-19) complicated with pneumonia in a patient with rheumatoid arthritis receiving conventional disease-modifying antirheumatic drugs. Rheumatol Int 40(6):991–995
Trivedi P, Patel SK, Bellavia D, Messina E, Palermo R, Ceccarelli S, Marchese C, Anastasiadou E, Minter LM, Felli MP (2021) When viruses cross developmental pathways. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2021.691644
Vaillancourt M, Jorth P (2020) The unrecognized threat of secondary bacterial infections with COVID-19. Mbio 11(4):e01806-01820
Vietzen H, Zoufaly A, Traugott M, Aberle J, Aberle S, Puchhammer-Stöckl E (2020) "NK cell receptor NKG2C deletion and HLA-E variants are risk factors for severe COVID-19. Genet Med 23(5):963–967
Wang F, Zheng S, Zheng C, Sun X (2020) Attaching clinical significance to COVID-19-associated diarrhea. Life Sci 260:118312
Wang LT, Liu KJ, Sytwu HK, Yen ML, Yen BL (2021) Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl Med 10(9):1288–1303
Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM (2010) A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 5(4):e10088
Westblade LF, Simon MS, Satlin MJ (2021) Bacterial co-infections in Coronavirus Disease 2019. Trends Microbiol 29(10):930–941
White IA, Sanina C, Balkan W, Hare JM (2016) Mesenchymal stem cells in cardiology. Mesenchymal stem cells. Springer, pp 55–87
Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, Van Doremalen N, Leighton I, Yinda CK, Pérez-Pérez L (2020) Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585(7824):273–276
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367(6483):1260–1263
Xaki S, Fathi A, Ariana M, Aghayan HR, Arjmand B, Alavi-Moghadam S, Azhdari K, Hazrati E (2021) Effects of adipose-derived mesenchymal stem cells and human amniotic membrane on sciatic nerve repair in rats. Arch Neurosci. https://doi.org/10.5812/ans.118661
Xu D, Qu C-K (2008) Protein tyrosine phosphatases in the JAK/STAT pathway. Front Biosci 13:4925–4932
Yagi H, Chen AF, Hirsch D, Rothenberg AC, Tan J, Alexander PG, Tuan RS (2020) Antimicrobial activity of mesenchymal stem cells against Staphylococcus aureus. Stem Cell Res Ther 11(1):293
Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y (2021) The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Target Ther 6(1):1–20
Yin Q, Wang L, Yu H, Chen D, Zhu W, Sun C (2021) Pharmacological effects of polyphenol phytochemicals on the JAK-STAT signaling pathway. Front Pharmacol. https://doi.org/10.3389/fphar.2021.71667
Yue J, López JM (2020) Understanding MAPK signaling pathways in apoptosis. Int J Mol Sci 21(7):2346
Zhao N, Di B, Xu L-L (2021) The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev 61:2–15
Zheng G, Ge M, Qiu G, Shu Q, Xu J (2015) Mesenchymal stromal cells affect disease outcomes via macrophage polarization. Stem Cells Int 2015:989473
Zhou Y-W, Xie Y, Tang L-S, Pu D, Zhu Y-J, Liu J-Y, Ma X-L (2021) Therapeutic targets and interventional strategies in COVID-19: mechanisms and clinical studies. Signal Transduct Target Ther 6(1):1–25
Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, Zhu F, Zhu B, Cui L (2020) Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res 285:198005
Zipeto D, Palmeira JDF, Argañaraz GA, Argañaraz ER (2020) ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front Immunol. https://doi.org/10.3389/fimmu.2020.5767
Zumla A, Wang F-S, Ippolito G, Petrosillo N, Agrati C, Azhar EI, Chang C, El-Kafrawy SA, Osman M, Zitvogel L (2020) Reducing mortality and morbidity in patients with severe COVID-19 disease by advancing ongoing trials of Mesenchymal Stromal (stem) Cell (MSC) therapy—achieving global consensus and visibility for cellular host-directed therapies. Elsevier 96:431–439
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design.SA-M, and MS wrote the first draft. MR-T, AR-M, and RA helped to study and gather information. EN-E extensively edited the manuscript. BL participated in a critical review. BA helped supervise the project and gave final approval of the version to be published. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arjmand, B., Alavi-Moghadam, S., Sarvari, M. et al. Critical roles of cytokine storm and bacterial infection in patients with COVID-19: therapeutic potential of mesenchymal stem cells. Inflammopharmacol 31, 171–206 (2023). https://doi.org/10.1007/s10787-022-01132-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01132-6