Abstract
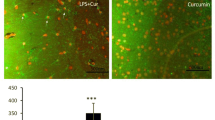
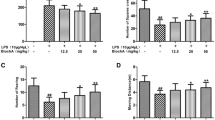
Various studies have indicated a lower incidence and prevalence of neurological conditions in people consuming curcumin. The ability of curcumin to target multiple cascades, simultaneously, could be held responsible for its neuroprotective effects. The present study was designed to investigate the potential of curcumin in minimizing microglia-mediated damage in lipopolysaccharide (LPS) induced model of PD. Altered microglial functions and increased inflammatory profile of the CNS have severe behavioral consequences. In the current investigation, a single injection of LPS (5 ug/5 µl PBS) was injected into the substantia nigra (SN) of rats, and curcumin [40 mg/kg b.wt (i.p.)] was administered daily for a period of 21 days. LPS triggered an inflammatory response characterized by glial activation [Iba-1 and glial fibrillary acidic protein (GFAP)] and pro-inflammatory cytokine production (TNF-α and IL-1β) leading to extensive dopaminergic loss and behavioral abnormality in rats. The behavioral observations, biochemical markers, quantification of dopamine and its metabolites (DOPAC and HVA) using HPLC followed by IHC of tyrosine hydroxylase (TH) were evaluated after 21 days of LPS injection. Curcumin supplementation prevented dopaminergic degeneration in LPS-treated animals by normalizing the altered levels of biomarkers. Also, a significant improvement in TH levels as well as behavioral parameters (actophotometer, rotarod, beam walking and grid walking tests) were seen in LPS injected rats. Curcumin shielded the dopaminergic neurons against LPS-induced inflammatory response, which was associated with suppression of glial activation (microglia and astrocytes) and transcription factor NF-κB as depicted from RT-PCR and EMSA assay. Curcumin also suppressed microglial NADPH oxidase activation as observed from NADPH oxidase activity. The results suggested that one of the important mechanisms by which curcumin mediates its protective effects in the LPS-induced PD model is by inhibiting glial activation. Therefore, curcumin could be a potential therapeutic agent for inflammation-driven neurodegenerative disorders like PD, and its neuroprotective role should be explored further.













Similar content being viewed by others
Change history
16 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10787-021-00886-9
Abbreviations
- AD:
-
Alzheimer’s disease
- DA:
-
Dopamine
- DCFH-DA:
-
2′-7′-Dichlorordihydrofluorescein diacetate
- GFAP:
-
Glial fibrillary acidic protein
- HD:
-
Huntington’s chorea
- IL-1β:
-
Interleukin-1β
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor-κB
- NADPH oxidase:
-
Nicotinamide adenine dinucleotide phosphate-oxidase
- NO:
-
Nitric oxide
- LPO:
-
Lipid peroxidation
- LPS:
-
Lipopolysaccharide
- PD:
-
Parkinson’s disease
- PHOX:
-
Phagocytic oxidase
- PMF:
-
Post-mitochondrial fraction
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SN:
-
Substantia nigra
- TH:
-
Tyrosine hydroxylase
- TNF-α:
-
Tumor necrosis factor-alpha
References
Aggarwal BB, Sundaram C, Malani N, Ichikawa H (2007) Curcumin: the Indian solid gold. Adv Exp Med Biol 595:1–75
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of Curcumin: problems and promises. Mol Pharm 4(6):807–818
Best TM (1999) Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J Appl Physiol 87:74–82
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69
Butler RN et al (2008) New model of health promotion and disease prevention for the 21st century. BMJ 337:a399
Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC (2006) CNS immune privilege: hiding in plain sight. Immunol Rev 213:48–65
Carter RJ, Morton AJ, Dunnett SB (2001) Current protocols in neuroscience, vol 15, pp 8.12.1–8.12.14
Chung ES, Bok E, Chung YC, Baik HH, Jin BK (2012) Cannabinoids prevent lipopolysaccharide induced neurodegeneration inthe rat substantia nigra in vivo throughinhibition of microglial activation and NADPH oxidase. Brain Res 1451:110–116
Church W (2005) Column chromatography analysis of brain tissue: an advanced laboratory exercise for neuroscience majors. J Undergrad Neurosci Educ 3:A36–A41
Cotrina ML, Nedergaard M (2002) Astrocytes in the aging brain. J Neurosci Res 67:1–10
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Deitch AD, Moses MJ (1957) The nissl substance of living and fixed spinal ganglion cells. J Biophys Biochem Cytol 3(23):449–456
Ding Y (1990) Impaired motor activity and motor learning function in rat with middle cerebral artery occlusion. Behav Brain Res 132:29–36
Finch CE (2003) Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging 24(1):S123–S131
Fischer R, Maier O (2015) Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxidative Medicine and Cellular Longevity. http://dx.doi.org/10.1155/2015/610813 (Article ID 610813)
Fu Y et al (2014) Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int Immunopharmacol 20:54–58
Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B (2002) Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem 81(6):1285–1297
Garman RH (2011) Histology of the central nervous system. Toxicol Pathol 39:22–35
Ginhoux F et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845
Godbout JP et al (2005) Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J 19:1329–1331
Gupta SC, Patchva S, Aggarwal BB (2012) Discovery of Curcumin, a component of the golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39(3):283–299
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
He LF, Chen HJ, Qian LH, Chen GY, Buzby JS (2010) Curcumin protects pre-oligodendrocytes from activated microglia in vitro and in vivo. Brain Res 21(1339):60–69
Herrera AJ, Castano A, Venero JL, Cano J, Machado A (2000) The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis 7(4):429–447
Holmes W (1947) In recent advances in clinical pathology. Churchill, London
Humason GL (1962) Basic procedures—animal tissue technique. Animal Tissue Techniques, Part-1. W. H. Freeman & Co., San Francisco, pp 130–132
Iravani MM, Leung CCM, Sadeghian M, Haddon CO, Rose S, Jenner P (2005) The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur J Neurosci 22(2):317–330
Jellinger KA (2000) Cell death mechanisms in Parkinson’s disease. J. Neural Transm 107:1–29
Kalonia H (2012) Targeting neuro-inflammatory cytokines and oxidative stress by minocycline attenuates quinolinic-acid-induced Huntington’s disease-like symptoms in rats. Neurotox Res 22:310–320
Knott C, Stern G, Wilkin GP (2000) Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci 16(6):724–739
Koppula S, Kumar H, Kim IS, Choi DK (2012) Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinson’s disease. Mediat Inflamm 2012. http://dx.doi.org/10.1155/2012/823902
Kulkarni SK (2000) Handbook of experimental pharmacology, 3rd edn. Vallabh Prakashan, Delhi
Lee J, Chan SL, Mattson MP (2002) Adverse effect of a presenilin-1 mutation in microglia results in enhanced nitric oxide and inflammatory cytokine responses to immune challenge in the brain. Neuromolecular Med 2(1):29–45
Lowry OH et al (1951) Protein measurement with the Follin’s phenol reagent. J Biol Che 193:265–275
McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG (1993) Microglia in degenerative neurological disease. Glia 7(1):84–92
Mirza B, Hadberg H, Thomsen P, Moos T (2000) The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience 95(2):425–432
Nagatsu T, Sawada M (2006) Cellular and molecular mechanisms of Parkinson’s disease: neurotoxins, causative genes, and inflammatory cytokines. Cell Mol Neurobiol 26:781–802
Nguyen MD, Julien JP, Rivest S (2002) Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 3:216–227
Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE (1993) GFAP mRNA increases with age in rat and human brain. Neurobiol Aging 14:421–429
Norden DM, Godbout JP (2013) Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 39(1):19–34
Pan MH, Huang TM, Lin JK (1999) Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 27:486–494
Paxinos GW, Watson C (2005) The rat brain in stereotaxic coordinates. Academic Press, Oxford
Pearse AGE (1969) Histochemical, theoretical and applied, 3rd edn. Churchill Livingstone, London, p 660
Puntambekar SS, Doose JM, Carson MJ (2008) Microglia: a CNS-specific tissue macrophage. In: Lane TE, Carson M, Bergmann C, Wyss-Coray T (eds) Central nervous system diseases and inflammation, 1st Edn. Springer, New York, pp 1–12
Raddassi K (1993) Role of calcium in the activation of mouse peritoneal macrophages: induction of NO synthase by calcium ionophores and thapsigargin. Cell Immunol 153:443–455
Ransohoff RM, Perry VH (2009) Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27:119–145
Ravindranath V, Chandrasekhara N (1980) Absorption and tissue distribution of curcumin in rats. Toxicology 16:259–265
Sharma N, Nehru B (2015) Characterization of the lipopolysaccharide induced model of Parkinson’s disease: role of oxidative stress and neuroinflammation. Neurochem Int 87:92–105
Sharma N, Nehru B (2016) Apocyanin, a microglial NADPH oxidase inhibitor prevents dopaminergic neuronal degeneration in lipopolysaccharide induced Parkinson’s disease model. Mol Neurobiol 53(5):3326–3337
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64(4):353–356
Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K (2007) Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55:412–424
Sikora E, Bielak-Zmijewska A, Mosieniak G, Piwocka K (2010a) The promise of slow down ageing may come from curcumin. Curr Pharmacol Des 16(7):884–892
Sikora E, Scapagnini G, Barbagallo M (2010b) Curcumin, inflammation, ageing and age-related diseases. Immun Ageing 7:1
Sly LM et al (2001) Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res Bull 56:581–588
Tansey MG, Wyss-Coray T (2008) Cytokines in CNS inflammation and disease. In: Lane TE, Carson M, Bergmann C, Wyss-Coray T (eds) Central nervous system diseases and inflammation, 1st Edn. Springer, New York, pp 59–106
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62:803–819
Unger JW (1998) Glial reaction in aging and Alzheimer’s disease. Microsc Res Tech 43:24–28
Vijg J, Campisi J (2008) Puzzles, promises and a cure for ageing. Nature 454:1065–1071
Vila M, Przedborski S (2004) Genetic clues to the pathogenesis of Parkinson’s disease. Nat Med 10:58–62
Whitton PS (2007) Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol 150(8):963–976
Wills ED (1996) Mechanism of lipid peroxide formation in animal tissue. Biochem J 99:667
Wood PL (2003) Microglia: roles of microglia in chronic neurodegenerative diseases. In: Wood PL (ed) Neuroinflammation: mechanisms and management. Totowa Humana Press, New York, pp 3–27
Zhu et al (2014) Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J Neuroinflamm 11:59
Acknowledgement
The study was carried out with the funds provided by Indian Council of Medical Research (ICMR) India (Grant No. 45/52/2013-PHA/BMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any competing interests in the manuscript.
Rights and permissions
About this article
Cite this article
Sharma, N., Sharma, S. & Nehru, B. Curcumin protects dopaminergic neurons against inflammation-mediated damage and improves motor dysfunction induced by single intranigral lipopolysaccharide injection. Inflammopharmacol 25, 351–368 (2017). https://doi.org/10.1007/s10787-017-0346-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0346-z