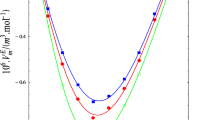
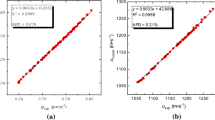
Abstract
Experimental densities ρ and sound speeds u at (293.15, 298.15, 303.15, 308.15, 313.15, and 318.15) K and refractive indices nD at 298.15 K are reported for the 1-propanol + cyclohexane, 1-propanol + cyclohexene, and 1-propanol + cyclohexanone binary mixtures covering the entire composition ranges and under atmospheric pressure. The excess molar volumes \({V}_{m}^{E}\), isentropic compressibility deviations \(\Delta {\kappa }_{S}\), and refractive index deviations ΔnD were derived from the experimental data. Redlich–Kister polynomial was the mathematical model of choice to correlate the derived properties of the studied mixtures. In each case, the Redlich–Kister polynomial with an optimal number of parameters provided a statistically significant mathematical representation of the derived properties with standard deviations compared to the estimated expanded uncertainties of corresponding properties. Furthermore, the Perturbed Chain Statistical Associating Fluid Theory (PC-SAFT) was used to correctly model the density of pure fluids and mixtures, whereas the coupling of PC-SAFT with Schaaff’s collision factor theory (SCFT) and Laplace mixing rules proved to be successful approaches for modeling the speed of sound and refractive index, respectively.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
N.G. Tsierkezos, I.E. Molinou, A.C. Filippou, J. Solut. Chem. 34, 1371 (2005). https://doi.org/10.1007/s10953-005-8508-9
B. González, N. Calvar, Á. Domínguez, J. Tojo, J. Chem. Thermodyn. 39, 322 (2007). https://doi.org/10.1016/j.jct.2006.06.008
O. Ciocirlan, M. Teodorescu, D. Dragoescu, O. Iulian, A. Barhala, J. Chem. Eng. Data 55, 968 (2010). https://doi.org/10.1021/je900404r
L. Lee, M. Chuang, J. Chem. Eng. Data 42, 850 (1997). https://doi.org/10.1021/je9603335
S.L. Oswal, K.D. Prajapati, J. Chem. Eng. Data 43, 367 (1998). https://doi.org/10.1021/je970235z
R.L. Gardas, S. Oswal, Thermochim. Acta 479, 17 (2008). https://doi.org/10.1016/j.tca.2008.09.006
R. Tanaka, T. Yokoyama, J. Solut. Chem. 33, 1061 (2004). https://doi.org/10.1023/B:JOSL.0000048056.49122.6d
T.M. Letcher, J. Mercer-Chalmers, Can. J. Chem. 69, 1259 (1991). https://doi.org/10.1139/v91-188
M. El-Hefnawy, K. Sameshima, T. Matsushita, R. Tanaka, J. Solut. Chem. 34, 43 (2005). https://doi.org/10.1007/s10953-005-2072-1
M.V.P. Rao, P.R. Naidu, J. Chem. Thermodyn. 8, 96 (1976). https://doi.org/10.1016/0021-9614(76)90157-9
D. NguyenHuynh, T.T. Nguyen, T.T.X. Nguyen, Fluid Phase Equilib. 434, 7 (2017). https://doi.org/10.1016/j.fluid.2016.11.020
M. Kleiner, G. Sadowski, J. Phys. Chem. C 111, 15544 (2007). https://doi.org/10.1021/jp072640v
J. Gross, G. Sadowski, Ind. Eng. Chem. Res. 40, 1244 (2001). https://doi.org/10.1021/ie0003887
F. Tumakaka, G. Sadowski, Fluid Phase Equilib. 217, 233 (2004). https://doi.org/10.1016/j.fluid.2002.12.002
D. Belhadj, A. Negadi, A. Hernández, I. Mokbel, I. Bahadur, L. Negadi, J. Chem. Thermodyn. 172, 106820 (2022). https://doi.org/10.1016/j.jct.2022.106820
W. Schaaffs, Acta Acust. United Acust. 33, 272 (1975)
B. Giner, C. Lafuente, A. Villares, M. Haro, M.C. López, J. Chem. Thermodyn. 39, 148 (2007). https://doi.org/10.1016/j.jct.2006.05.003
N.L. Benkelfat-Seladji, F. Ouaar, A. Hernández, N. Muñoz-Rujas, I. Bahadur, N. Chiali-Baba Ahmed, E. Montero, L. Negadi, J. Chem. Eng. Data 66, 3397 (2021). https://doi.org/10.1021/acs.jced.1c00131
N.L. Benkelfat-Seladji, F. Ouaar, A. Hernández, I. Bahadur, N. Muñoz-Rujas, S.K. Singh, E. Montero, N. Chiali-Baba Ahmed, L. Negadi, J. Chem. Thermodyn. 170, 106762 (2022). https://doi.org/10.1016/j.jct.2022.106762
T. Arbneshi, A. Qerimi, A. Zeqiraj, N. Syla, F.R. Aliaj, J. Chem. Eng. Data 67, 2098 (2022). https://doi.org/10.1021/ACS.JCED.2C00093
F. Aliaj, N. Syla, A. Kurtishaj, N. Elezaj, Z. Tolaj, T. Arbneshi, A. Zeqiraj, Int. J. Thermophys. 41, 49 (2020). https://doi.org/10.1007/s10765-020-02632-9
J. Ortega, J. Chem. Eng. Data 27, 312 (1982). https://doi.org/10.1021/je00029a024
J.L. Hales, J.H. Ellender, J. Chem. Thermodyn. 8, 1177 (1976). https://doi.org/10.1016/0021-9614(76)90126-9
B. Marrufo, S. Loras, M. Sanchotello, J. Chem. Eng. Data 55, 5812 (2010). https://doi.org/10.1021/je100776x
G. Tardajos, M. Diaz Pena, A. Lainez, E. Aicart, J. Chem. Eng. Data 31, 492 (1986). https://doi.org/10.1021/je00046a031
I. Domínguez, N. Calvar, E. Gómez, Á. Domínguez, J. Chem. Thermodyn. 43, 705 (2011). https://doi.org/10.1016/j.jct.2010.12.012
T.M. Letcher, J. Chem. Thermodyn. 9, 661 (1977). https://doi.org/10.1016/0021-9614(77)90091-X
C. Bermúdez-Salguero, J. Gracia-Fadrique, E. Calvo, A. Amigo, J. Chem. Eng. Data 56, 3823 (2011). https://doi.org/10.1021/je200468r
K. Tamura, A. Osaki, Thermochim. Acta 352–353, 11 (2000). https://doi.org/10.1016/S0040-6031(99)00430-X
J.N. Nayak, M.I. Aralaguppi, T.M. Aminabhavi, J. Chem. Eng. Data 48, 628 (2003). https://doi.org/10.1021/je0201828
A. Zeqiraj, A. Gjevori, A. Llozana, N. Syla, F. Aliaj, Int. J. Thermodyn. 26, 48 (2022). https://doi.org/10.5541/ijot.1173589
P.R. Garrett, J.M. Pollock, K.W. Morcom, J. Chem. Thermodyn. 5, 569 (1973). https://doi.org/10.1016/S0021-9614(73)80105-3
F. Aliaj, A. Zeqiraj, Phys. Chem. Liq. (2023). https://doi.org/10.1080/00319104.2023.2188213
F. Aliaj, A. Bytyqi-Damoni, N. Syla, AKTET J. Inst. Alb-Shkenca 9, 36 (2016)
P. Brocos, Á. Piñeiro, R. Bravo, A. Amigo, Phys. Chem. Chem. Phys. 5, 550 (2003). https://doi.org/10.1039/b208765k
O. Redlich, A.T. Kister, Ind. Eng. Chem. 40, 345 (1948). https://doi.org/10.1021/ie50458a036
P.R. Bevington, D.K. Robinson, Data Reduction and Error Analysis for the Physical Sciences, 3rd edn. (McGraw-Hill, New York, 2003)
T.E. Daubert, R.P. Danner, Physical and Thermodynamic Properties of Pure Chemicals: Data Compilation (Taylor and Francis, Bristol, 2004)
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
AH performed modeling of physical properties, wrote Sect. 2, and prepared Figs. 4, 5, 6, and S3–S6; AZZ measured properties of the studied mixtures and prepared manuscript tables; FRA assisted in measurements, wrote the main manuscript text, and prepared Figs. 1, 2, 3, and S1–S2. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernández, A., Zeqiraj, A.Z. & Aliaj, F.R. Densities, Sound Speeds, and Refractive Indices of 1-Propanol + Cyclohexane (or Cyclohexene or Cyclohexanone) Binary Mixtures at Various Temperatures Under Atmospheric Pressure: Experimental and Modeling Study. Int J Thermophys 44, 102 (2023). https://doi.org/10.1007/s10765-023-03211-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03211-4