Abstract
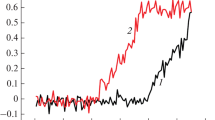
Nanoparticles are termed as kinetic promoters with great application potential whose promotion effect exceeding that of surfactants in some cases. However, the effects on hydrate dissociation in nanoparticle system have been studied rarely. In the presence of nanoparticles, there are differences in the heat conduction of different systems, which may cause different hydrate dissociation behaviors. Herein, the dissociation kinetics under different systems were quantitatively characterized using gas production and activity energy. It was found that silica nanoparticle promotes the CO2 hydrate dissociation and the gas production increases with the increase of nanoparticle mass fraction. The activation energy in different silica nanoparticle systems of 50 nm 0.05–1.00 wt% increases with the decrease of nanoparticle concentration with the value changing from 72.05 ± 4.21 kJ·mol−1 to 122.25 ± 11.46 kJ·mol−1. Compared with pure carbon dioxide hydrate, the thermal conductivity of CO2 hydrate-silica nanoparticle systems increase by 2.4 % to 5.5 %. The thermal conductivity increases with the increase in concentration or the decrease in particle size. There is an approximately linear negative correlation between activation energy and thermal conductivity, that is, as the thermal conductivity increases, the activation energy decreases. The law of fluctuation and dissipation is the internal mechanism of hydrate dissociation and that was analyzed firstly in macroscopic experimental investigation. It was found that the fluctuation and dissipation of gas production in the near-equilibrium region can be used to predict the hydrate dissociation kinetics under non-equilibrium conditions.













Similar content being viewed by others
References
E.D. Sloan, C.A. Koh, Clathrate Hydrates of Natural Gases (Chemical Industries Series), 3rd edn. (CRC Press, Boca Raton, 2007)
H. Zhou, I.D. Sera, C.I. Ferreira, Appl. Energy 158, 433 (2015). https://doi.org/10.1016/j.apenergy.2015.08.092
X.L. Wang, D. Mike, Chem. Eng. Sci. 155, 294 (2016). https://doi.org/10.1016/j.ces.2016.08.020
S.P. Kang, H. Lee, C.S. Lee, W.M. Sung, Fluid Phase Equilib. 185, 101 (2001). https://doi.org/10.1016/s0378-3812(01)00460-5
H. Yang, Z. Xu, M. Fan, R. Gupta, R.B. Slimane, A.E. Bland, I. Wright, J. Environ. Sci. 20, 14 (2008). https://doi.org/10.1016/s1001-0742(08)60002-9
Z. Ma, P.G. Ranjith, Fuel 255, 155644 (2019). https://doi.org/10.1016/j.fuel.2019.115644
K.C. Kang, P. Linga, K.N. Park, S.J. Choi, J.D. Lee, Desalination 353, 84 (2014). https://doi.org/10.1016/j.desal.2014.09.007
H. Matsui, J. Jia, T. Tsuji, Y. Liang, Y. Masuda, Fuel 263, 116640 (2020). https://doi.org/10.1016/j.desal.2014.09.007
O. Mahian, E. Bellos, C.N. Markides, R.A. Taylor, A. Alagumalai, L. Yang, C. Qin, B.J. Lee, G. Ahmadi, M.R. Safaei, S. Wongwises, Nano Energy 86, 106069 (2021). https://doi.org/10.1016/j.nanoen.2021.106069
O. Nashed, B. Partoon, B. Lal, K.M. Sabil, A.M. Shariff, J. Nat. Gas Sci. Eng. 55, 452 (2018). https://doi.org/10.1016/j.jngse.2018.05.022
X. Huang, G. Ma, P. Wang, Pet. Sci. Technol. (2021). https://doi.org/10.1080/10916466.2021.1967387
A.G. Aregbe, B. Sun, L. Chen, J. Chem. Eng. Data. 64, 2929 (2019). https://doi.org/10.1021/acs.jced.8b01173
M. Yang, J. Zhao, J. Zheng, Y. Song, Appl. Energy 256, 113878 (2019). https://doi.org/10.1016/j.apenergy.2019.113878
N. Adibi, M. Mohammadi, M.R. Ehsani, E. Khanmohammadian, J. Nat. Gas Sci. Eng. 84, 103690 (2020). https://doi.org/10.1016/j.jngse.2020.103690
P. Linga, C. Haligva, S.C. Nam, J.A. Ripmeester, P. Englezos, Energy Fuels 23, 5496 (2009). https://doi.org/10.1021/ef900542m
W.X. Pang, W.Y. Xu, C.Y. Sun, C.L. Zhang, G.J. Chen, Fuel 88, 497 (2009). https://doi.org/10.1016/j.fuel.2008.11.002
G.D. Holder, P.F. Angert, V.T. John, S. Yen, J. Pet. Technol. 34, 1127 (1982). https://doi.org/10.2118/8929-pa
D.L. Li, H. Peng, D.Q. Liang, Int. J. Heat Mass Transfer. 104, 566 (2017). https://doi.org/10.1016/j.ijheatmasstransfer.2016.08.081
X.Y. Li, X.S. Li, Y. Wang, J.W. Liu, H.Q. Hu, Energy 202, 117690 (2020). https://doi.org/10.1016/j.energy.2020.117690
X. Kou, Y. Wang, X.S. Li, Y. Zhang, Z.Y. Chen, Appl. Energy 251, 113405 (2019). https://doi.org/10.1016/j.apenergy.2019.113405
X.Y. Li, Y. Wang, X.S. Li, Y. Zhang, Z.Y. Chen, Int. J. Heat Mass Transfer. 144, 118528 (2019). https://doi.org/10.1016/j.ijheatmasstransfer.2019.118528
G.C. Song, Y.X. Li, W.C. Wang, K. Jiang, Z. Shi, X. Ye, P.F. Zhao, J. Nat. Gas Sci. Eng. 45, 26 (2017). https://doi.org/10.1016/j.jngse.2017.04.032
V.A. Vlasov, Chem. Eng. Sci. 215, 115443 (2020). https://doi.org/10.1016/j.ces.2019.115443
N.J. English, E.T. Clarke, J. Chem. Phys. 139, 094701 (2013). https://doi.org/10.1063/1.4819269
M.R. Ghaani, N.J. English, J. Chem. Phys. 148, 114504 (2018). https://doi.org/10.1063/1.5018192
M.R. Ghaani, N.J. English, Mol. Phys. 117, 2434 (2019). https://doi.org/10.1080/00268976.2019.1567845
J. Li, Z.L. Wang, Phys. Chem. Chem. Phys. 21, 23492 (2019). https://doi.org/10.1039/c9cp04780h
M.F. Fakoya, S.N. Shah, Petroleum 3, 391 (2017). https://doi.org/10.1016/j.petlm.2017.03.001
Z.L. Wang, D.W. Tang, S. Liu, X.H. Zheng, N. Araki, Int. J. Thermophys. 28, 1255 (2007). https://doi.org/10.1007/s10765-007-0254-3
V.D. Chari, D. Sharma, P. Prasad, S.R. Murthy, J. Nat. Gas Sci. Eng. 11, 7 (2013). https://doi.org/10.1016/j.jngse.2012.11.004
E. Chaturvedi, K. Patidar, S. Laik, A. Mandal, Mar. Georesour. Geotechnol. 37, 57 (2019). https://doi.org/10.1080/1064119x.2018.1443181
D.Y. Peng, D.B. Robinson, Ind. Eng. Chem. Fundam. 15, 59 (1976). https://doi.org/10.1021/i160057a011
R.G. Ross, P. Andersson, G. Bäckström, Nature 290, 322 (1981). https://doi.org/10.1038/290322a0
G. Yao, Study on thermal physical properties of gas hydrate, China University of Petroleum (East China), (2015). www.cnki.net
D.W. Tang, Z.L. Wang, Characterization of Thermophysical Properties of Micro-Nano Materials and Structures (Science Press, Beijing, 2010)
Y.M. Xuan, Sci. Sin. Technol. 44, 269 (2014). https://doi.org/10.1360/092013-1236
X. Zhou, Z. Long, S. Liang, Y. He, L. Yi, D. Li, D. Liang, Energy Fuels 30, 1279 (2016). https://doi.org/10.1021/acs.energyfuels.5b02119
N.J. English, G.M. Phelan, J. Chem. Phys. 131, 074704 (2009). https://doi.org/10.1063/1.3211089
E.M. Myshakin, H. Jiang, R.P. Warzinski, K.D. Jordan, J. Phys. Chem. A 113, 1913 (2009). https://doi.org/10.1021/jp807208z
C. Sun, C. Ma, G. Chen, T. Guo, J. Univ. Pet. (China) 25(3), 8 (2001)
M.A. Clarke, P.R. Bishnoi, Chem. Eng. Sci. 59, 2983 (2004). https://doi.org/10.1016/j.ces.2004.04.030
Q.C. Wan, L.L. Chen, B. Li, K. Peng, Y.Q. Wu, Ind. Eng. Chem. Res. 59, 10651 (2020). https://doi.org/10.1021/acs.iecr.0c00705
Acknowledgements
We acknowledge funding supports from National Natural Science Foundation of China (No. 51876223), Natural Science Foundation of Shandong Province (No. ZR201807060413)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiao, Lj., Wan, Rc. & Wang, Zl. Experimental Investigation of CO2 Hydrate Dissociation in Silica Nanoparticle System with Different Thermal Conductivity. Int J Thermophys 42, 170 (2021). https://doi.org/10.1007/s10765-021-02920-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-021-02920-y